Molecular oxygen
Molecular oxygen (O2) is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. Molecular oxygen is essential for life, as it is used for respiration by many organisms. It's also essential for fossil fuel combustion.
Molecular oxygen is very chemically reactive, and tends to form oxides by reaction with other elements and compounds quite easily. We rely on photosynthesis of plants to replenish the molecular oxygen in the atmosphere - if photosynthesis stopped, eventually the atmospheric oxygen content would drop to near zero.
Since animals (including humans) breathe molecular oxygen and require it for metabolism, it is important medically. Molecular oxygen is provided therapeutically in oxygen therapy and hyperbaric chambers, and is also included in breathing gas for space exploration and SCUBA diving.
Industrially, oxygen is used to remove sulfur and carbon impurities during smelting. Oxygen (as a compressed gas) is also used widely in welding and metal cutting, and as an oxidizer in rocket engines. Oxygen is also important in creating many chemical feedstocks, such as ethylene oxide. [2]
Properties
Some physical properties of molecular oxygen:
| Chemical formula | O2 |
| Molar mass | 32.00 g/mol[3] |
| Boiling Point | -183oC[4] |
| Melting Point | −219°C[4] |
Combustion
Molecular oxygen is important for combustion - especially in the combustion of fuels for energy. Combustion is the reaction of a compound (the fuel) with an oxidant (which is usually molecular oxygen) to produce oxides. The third component of combustion is a "boost" of energy needed in order to start the reaction - the activation energy. All three components (fuel, oxidant, energy) must be present for the combustion reaction to occur.
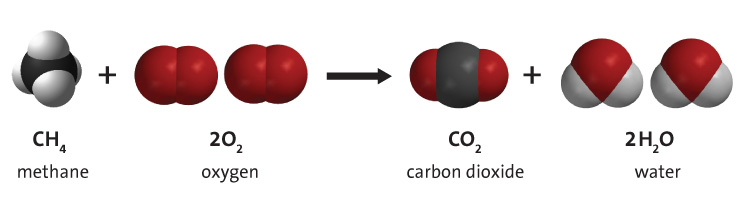
Combustion of fossil fuels such as methane, shown in Figure 2 below, produces carbon dioxide, water vapour, and energy. Fossil fuel combustion supplies around 95% of the world's primary energy. Hydrocarbon combustion also contributes to climate change by producing emissions such as carbon dioxide.
While other substances (such as fluorine) can act as oxidizers in combustion[6], oxygen is the most common. Oxidizers support combustion by accepting electrons from the fuel molecule, making it easier to break the chemical bonds in the reaction. While fluorine is actually a better electron acceptor than oxygen, oxidation by fluorine is too violent to be useful for most applications. Oxygen is also more abundant and easier to find, making it the most common oxidizer for these reactions.
References
- ↑ Wikimedia Commons. (May 12, 2015). Dioxygen [Online]. Available: http://commons.wikimedia.org/wiki/File:Dioxygen-3D-vdW.png#/media/File:Dioxygen-3D-vdW.png
- ↑ WebElements. (May 12, 2015). Oxygen [Online]. Available: https://www.webelements.com/oxygen/uses.html
- ↑ Web QC. (May 13, 2015). Molar Mass of O2 [Online]. Available: http://www.webqc.org/molecular-weight-of-O2.html
- ↑ 4.0 4.1 Royal Society of Chemistry. (May 12, 2015). Molecular Oxygen [Online]. Available: http://www.chemspider.com/Chemical-Structure.952.html
- ↑ American Chemical Society. (June 8, 2015). "Methane and oxygen react" [Online]. Available: http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1
- ↑ Sunner, Månsson (Eds.), "Experimental Chemical Thermodynamics, Vol. 1: Combustion Calorimetry" 1979, International Union of Pure and Applied Chemistry.

