Butane: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[category:fuels]] | [[category:fuels]] | ||
[[Category:Done | [[Category:Done 2018-06-15]] | ||
[[File:256px-Butane_torch.jpg|right|framed|Figure 1. A butane torch for kitchen use (specifically for [http://www.ricardocuisine.com/recipes/4016-creme-brulee crème brûlée]).<ref name=book1> [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons accessed 1/29/2015.</ref>]] | <translate> | ||
<!--T:1--> | |||
[[File:256px-Butane_torch.jpg|right|200px|framed|Figure 1. A butane torch for kitchen use (specifically for [http://www.ricardocuisine.com/recipes/4016-creme-brulee crème brûlée]).<ref name=book1> [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons accessed 1/29/2015.</ref>]] | |||
<onlyinclude>'''Butane''' is | <!--T:2--> | ||
<onlyinclude>'''Butane''' is an an alkane with the chemical formula C<sub>4</sub>H<sub>10</sub>. As a type of [[hydrocarbon]], it can undergo [[hydrocarbon combustion]] which gives off heat energy.</onlyinclude> Butane is one of the [[hydrocarbon]] components of raw [[natural gas]], which is a type of [[fossil fuel]].<ref>“NATURAL GAS FAQs,” Pacific Northern Gas RSS. [Online]. Available: http://www.png.ca/natural-gas-faqs/. [Accessed: 24-May-2017]</ref> Butane is usually removed from natural gas before being shipped to customers, but then butane is sold separately as a fuel itself. | |||
<!--T:3--> | |||
< | Butane is commonly mixed with [[propane]] in camping fuel in order to maintain higher [[pressure]]s at low [[temperature]]s.<ref name=book2>MSR.(2014). ''MSR ISOPRO - Performance Boosting Fuel for your Canister Stove'' [Online]. Available: http://www.cascadedesigns.com/msr/stoves/stove-accessories/msr-isopro/product [February 16, 2015].</ref> Butane is also one of the main components in lighter fluid and is commonly used in cigarette lighters, portable stoves and butane torches. Figure 1 shows a butane torch used for cooking purposes. | ||
==Properties== | ==Properties== <!--T:4--> | ||
[[File:Butane-3D-space-filling.png|thumb| | <!--T:5--> | ||
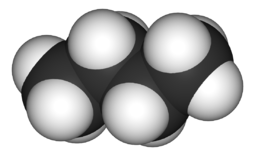
[[File:Butane-3D-space-filling.png|thumb|255px|Figure 2. Space filling model of butane, the white spheres represent[[hydrogen]] and the black spheres represent [[carbon]].<ref>"Butane-3D-space-filling". Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Butane-3D-space-filling.png#mediaviewer/File:Butane-3D-space-filling.png</ref>]] | |||
Below is a table of some of the basic properties of butane. | Below is a table of some of the basic properties of butane. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 25: | Line 29: | ||
|} | |} | ||
==Combustion | ==Combustion Reaction== <!--T:6--> | ||
Butane | |||
<html><iframe src=' | <!--T:7--> | ||
Butane releases its [[chemical energy]] by undergoing [[hydrocarbon combustion]]. Below is a [[hydrocarbon combustion]] animation showing the net reaction that occurs when butane combines with [[oxygen]]. | |||
<!--T:8--> | |||
<center> 2 (C<sub>4</sub>H<sub>10</sub>) + 13 (O<sub>2</sub>) → 8(CO<sub>2</sub>) + 10(H<sub>2</sub>O) + Heat Energy ([[Enthalpy]]) </center> | |||
<!--T:9--> | |||
<html><iframe src='https://energyeducation.ca/simulations/combustion/combustion_butane.html' width='900px' height='330px' style='border:none;position:relative;left:-35px'></iframe></html> | |||
<!--T:10--> | |||
The [[hydrocarbon combustion]] reaction releases [[heat]] [[energy]] and is an example of an [[exothermic reaction]]. The reaction also has a negative [[enthalpy]] change (ΔH) value. | |||
==For Further Reading== | |||
*[[Chemical Energy]] | |||
*[[Chemical bond]] | |||
*[[Combustion]] | |||
*[[Primary energy]] | |||
*[[Energy conversion technology]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
</translate> | |||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
<languages /> | |||
Revision as of 21:08, 24 June 2018
<translate>

Butane is an an alkane with the chemical formula C4H10. As a type of hydrocarbon, it can undergo hydrocarbon combustion which gives off heat energy. Butane is one of the hydrocarbon components of raw natural gas, which is a type of fossil fuel.[2] Butane is usually removed from natural gas before being shipped to customers, but then butane is sold separately as a fuel itself.
Butane is commonly mixed with propane in camping fuel in order to maintain higher pressures at low temperatures.[3] Butane is also one of the main components in lighter fluid and is commonly used in cigarette lighters, portable stoves and butane torches. Figure 1 shows a butane torch used for cooking purposes.
Properties
Below is a table of some of the basic properties of butane.
| Formula | C4H10 |
| Molar mass | 58.12 grams/mole |
| Energy density | 49.5 MJ/kg[5] |
| Melting Point | -138oC[6] |
| Boiling Point | -0.5oC[6] |
Combustion Reaction
Butane releases its chemical energy by undergoing hydrocarbon combustion. Below is a hydrocarbon combustion animation showing the net reaction that occurs when butane combines with oxygen.
The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
For Further Reading
- Chemical Energy
- Chemical bond
- Combustion
- Primary energy
- Energy conversion technology
- Or explore a random page
References
</translate>
- ↑ [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0) or GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons accessed 1/29/2015.
- ↑ “NATURAL GAS FAQs,” Pacific Northern Gas RSS. [Online]. Available: http://www.png.ca/natural-gas-faqs/. [Accessed: 24-May-2017]
- ↑ MSR.(2014). MSR ISOPRO - Performance Boosting Fuel for your Canister Stove [Online]. Available: http://www.cascadedesigns.com/msr/stoves/stove-accessories/msr-isopro/product [February 16, 2015].
- ↑ "Butane-3D-space-filling". Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Butane-3D-space-filling.png#mediaviewer/File:Butane-3D-space-filling.png
- ↑ Glenn Elert. (2015). The Physics Hypertextbook - Chemical Potential Energy [Online]. Available: http://physics.info/energy-chemical/ [February 16, 2015].
- ↑ 6.0 6.1 Charles E. Ophardt. (2003). Virtual Chembook - Hydrocarbon Boiling Points [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html [February 16,2015].
<languages />