Hydrocarbon: Difference between revisions
m (1 revision imported) |
energy>Ethan.boechler No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
<onlyinclude>The term '''hydrocarbon''' refers to the most basic type of [[organic molecule]]s. | [[Category:Translated to Spanish]] | ||
[[es:Hidrocarburo]] | |||
[[Category:Translated to French]] | |||
[[fr:Hydrocarbure]] | |||
<onlyinclude>The term '''hydrocarbon''' refers to the most basic type of [[organic molecule]]s. As suggested by their name, they are comprised of only 2 elements: [[hydrogen]] and [[carbon]].<ref name="hyper1">R. Nave. "Hydrocarbons." Internet: http://hyperphysics.phy-astr.gsu.edu/hbase/organic/hydrocarbon.html , [October 25, 2013].</ref> Hydrocarbon [[molecule]]s have one or more central carbon [[atom]]s in a branched or chain-like structure, surrounded by hydrogen atoms. </onlyinclude> There are four main categories of hydrocarbons: [[Alkane]]s, [[Alkene]]s, [[Alkyne]]s, and [[Aromatic hydrocarbon]]s.<ref>"Hydrocarbons - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Core/Organic_Chemistry/Hydrocarbons. [Accessed: 07- Jun- 2018].</ref> | |||
The simplest hydrocarbons are called [[alkane]]s. Alkanes are | ==Structure== | ||
The simplest hydrocarbons are called [[alkane]]s. Alkanes are made exclusively with '''single bonds''' between the carbon atoms. Figure 1 shows some small alkane molecules - notice that all the C-C bonds are single bonds. This means that all carbons in an alkane have a tetrahedral geometry. The examples in Figure 1 are all "straight chain" alkanes, where the central carbons form a single linear chain. Alkanes may also be branched <ref>"Branched Alkanes - Chemistry LibreTexts", chem.libretexts.org, 2019. [Online]. Available: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.03%3A_Branched_Alkanes . [Accessed: 14-Jun-2019]. </ref> or cyclic <ref>"Cyclic Hydrocarbons - Chemistry LibreTexts", chem.libretexts.org, 2019. [Online]. Available:https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.06%3A_Cyclic_Hydrocarbons. [Accessed: 14-Jun-2019]. </ref>, and can contain any number of carbon atoms. | |||
[[File:Alkanes.jpg|450px|thumbnail|right|Figure 1 The smallest hydrocarbons.<ref name="image">D. Darling. "Alkanes." Internet: http://www.daviddarling.info/encyclopedia/A/alkane.html, [October 26, 2013]</ref>]] | [[File:Alkanes.jpg|450px|thumbnail|right|Figure 1 The smallest hydrocarbons.<ref name="image">D. Darling. "Alkanes." Internet: http://www.daviddarling.info/encyclopedia/A/alkane.html, [October 26, 2013]</ref>]] | ||
Similar in structure to alkanes, [[alkene]]s are hydrocarbons where the central carbon chain contains at least one double bond between carbon atoms, and [[alkyne]]s contain at least one triple bond. Also commonly seen are [[hydrocarbon derivative]]s, where atoms other than carbon or hydrogen are present.<ref name="hyper1"></ref> For example in an '''alkyl halide''', a hydrogen from an alkane is replaced by a [[halogen]] atom such as chlorine or bromine. | |||
Hydrocarbons and their derivatives are the main constituents of [[fossil fuel]]s, and release [[energy]] through [[Hydrocarbon combustion | combustion]]. Besides their [[fuel]] applications, hydrocarbons are also used in chemical synthesis and are major components of lubricating oils, greases, solvents, fuels, wax, asphalts, cosmetics, and plastics.<ref name="challenge">R.D. Botts, D.M. Carson, and D.Coglon. "Petroleum in our live" in ''Our petroleum challenge'', 8th ed. Calgary:Canadian Center for Energy Development, 2013, pp. 7-15.</ref> These non-fuel applications of hydrocarbons can be of great importance to society and the economy. | |||
==Sources== | |||
The main source for hydrocarbons is through [[fractional distillation]] of fossil fuels, especially [[petroleum]]. However, researchers are investigating alternate and renewable sources, such as converting used frying oil (usually plant-based) to biodiesel <ref>"Used and Waste Oil and Grease for Biodiesel", J. Van Gerpen, 2019. [Online]. Available: https://farm-energy.extension.org/used-and-waste-oil-and-grease-for-biodiesel/. [Accessed: 14-Jun-2019]. </ref>, or reclaiming waste oils from industrial processes <ref>"Forging New Sources for Biofuels and Hydrocarbon-Based Products", albertainnovates.ca, 2018. [Online] Available: https://albertainnovates.ca/project/forging-new-sources-for-biofuels-and-hydrocarbon-based-products/, [Accessed: 14-Jun-2019]. </ref> | |||
To learn more about Hydrocarbons, check out the [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons UC Davis Chem Wiki]. | To learn more about Hydrocarbons, check out the [http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons UC Davis Chem Wiki]. | ||
Revision as of 20:57, 10 September 2021
The term hydrocarbon refers to the most basic type of organic molecules. As suggested by their name, they are comprised of only 2 elements: hydrogen and carbon.[1] Hydrocarbon molecules have one or more central carbon atoms in a branched or chain-like structure, surrounded by hydrogen atoms. There are four main categories of hydrocarbons: Alkanes, Alkenes, Alkynes, and Aromatic hydrocarbons.[2]
Structure
The simplest hydrocarbons are called alkanes. Alkanes are made exclusively with single bonds between the carbon atoms. Figure 1 shows some small alkane molecules - notice that all the C-C bonds are single bonds. This means that all carbons in an alkane have a tetrahedral geometry. The examples in Figure 1 are all "straight chain" alkanes, where the central carbons form a single linear chain. Alkanes may also be branched [3] or cyclic [4], and can contain any number of carbon atoms.
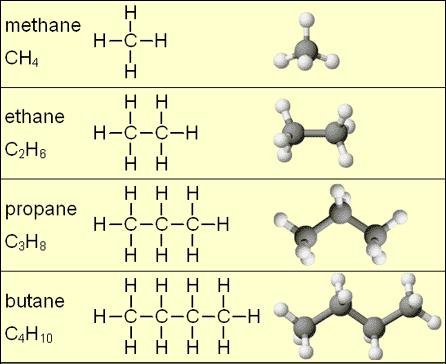
Similar in structure to alkanes, alkenes are hydrocarbons where the central carbon chain contains at least one double bond between carbon atoms, and alkynes contain at least one triple bond. Also commonly seen are hydrocarbon derivatives, where atoms other than carbon or hydrogen are present.[1] For example in an alkyl halide, a hydrogen from an alkane is replaced by a halogen atom such as chlorine or bromine.
Hydrocarbons and their derivatives are the main constituents of fossil fuels, and release energy through combustion. Besides their fuel applications, hydrocarbons are also used in chemical synthesis and are major components of lubricating oils, greases, solvents, fuels, wax, asphalts, cosmetics, and plastics.[6] These non-fuel applications of hydrocarbons can be of great importance to society and the economy.
Sources
The main source for hydrocarbons is through fractional distillation of fossil fuels, especially petroleum. However, researchers are investigating alternate and renewable sources, such as converting used frying oil (usually plant-based) to biodiesel [7], or reclaiming waste oils from industrial processes [8]
To learn more about Hydrocarbons, check out the UC Davis Chem Wiki.
For Further reading
Check out the specific types of hydrocarbons:
References
- ↑ 1.0 1.1 R. Nave. "Hydrocarbons." Internet: http://hyperphysics.phy-astr.gsu.edu/hbase/organic/hydrocarbon.html , [October 25, 2013].
- ↑ "Hydrocarbons - Chemistry LibreTexts", Chem.libretexts.org, 2018. [Online]. Available: https://chem.libretexts.org/Core/Organic_Chemistry/Hydrocarbons. [Accessed: 07- Jun- 2018].
- ↑ "Branched Alkanes - Chemistry LibreTexts", chem.libretexts.org, 2019. [Online]. Available: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.03%3A_Branched_Alkanes . [Accessed: 14-Jun-2019].
- ↑ "Cyclic Hydrocarbons - Chemistry LibreTexts", chem.libretexts.org, 2019. [Online]. Available:https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.06%3A_Cyclic_Hydrocarbons. [Accessed: 14-Jun-2019].
- ↑ D. Darling. "Alkanes." Internet: http://www.daviddarling.info/encyclopedia/A/alkane.html, [October 26, 2013]
- ↑ R.D. Botts, D.M. Carson, and D.Coglon. "Petroleum in our live" in Our petroleum challenge, 8th ed. Calgary:Canadian Center for Energy Development, 2013, pp. 7-15.
- ↑ "Used and Waste Oil and Grease for Biodiesel", J. Van Gerpen, 2019. [Online]. Available: https://farm-energy.extension.org/used-and-waste-oil-and-grease-for-biodiesel/. [Accessed: 14-Jun-2019].
- ↑ "Forging New Sources for Biofuels and Hydrocarbon-Based Products", albertainnovates.ca, 2018. [Online] Available: https://albertainnovates.ca/project/forging-new-sources-for-biofuels-and-hydrocarbon-based-products/, [Accessed: 14-Jun-2019].

