Infrared radiation: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
2dev>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-12-10]] [[Category: Rudi grade Ashley edit]] | ||
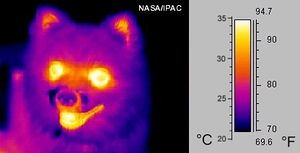
[[File:Infrared_dog.jpg|300px|thumb|Figure 1. A dog as seen in the infrared spectrum.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/0/0c/Infrared_dog.jpg</ref>]] | [[File:Infrared_dog.jpg|300px|thumb|Figure 1. A dog as seen in the infrared spectrum.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/0/0c/Infrared_dog.jpg</ref>]] | ||
| Line 5: | Line 5: | ||
==Effect of IR== | ==Effect of IR== | ||
Even though infrared radiation cannot be seen, it can definitely be felt. Infrared energy is felt as [[heat]] because it interacts with [[molecule]]s by exciting them, causing them to move | Even though infrared radiation cannot be seen by the human eye, it can definitely be felt. Infrared energy is felt as [[heat]] because it interacts with [[molecule]]s by exciting them, causing them to move faster which increases the internal [[temperature]] of the object absorbing the infrared energy. Although all wavelengths of radiant energy will heat surfaces that absorb them, infrared radiation is most common in daily life because of the "ordinary" objects that emit it as [[radiant heat]] (see [[blackbody radiation]] and [[Wien's Law]] for more information on this).<ref>Hyperphysics, ''Heat Radiation'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html#c2</ref> For example, humans at a temperature of [[celsius|37°C]]<ref name=yea>R. A. Hinrichs and M. Kleinbach, "Heat and Work," in ''Energy: Its Use and the Environment'', 4th ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.111-114</ref> emit most of their radiant heat in the infrared range, as can be seen in Figure 1. | ||
[[File:co2gif.gif|318px|thumb|Figure 2. Carbon dioxide is able to interact with infrared radiation, leading to an imbalance of radiation entering and leaving the atmosphere.<ref>PhET Simulations, ''Molecules and Light'' [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light</ref>]] | [[File:co2gif.gif|318px|thumb|Figure 2. Carbon dioxide is able to interact with infrared radiation, leading to an imbalance of radiation entering and leaving the atmosphere.<ref>PhET Simulations, ''Molecules and Light'' [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light</ref>]] | ||
Around 50% of the [[solar energy to the Earth|Sun's energy to the Earth]] is in the form of infrared,<ref>Passive heating and cooling manual, ''Introduction to Solar Energy'' [Document], Available: http://www.azsolarcenter.com/design/documents/passive.DOC</ref> therefore the balance of this radiation in | Around 50% of the [[solar energy to the Earth|Sun's energy to the Earth]] is in the form of infrared,<ref>Passive heating and cooling manual, ''Introduction to Solar Energy'' [Document], Available: http://www.azsolarcenter.com/design/documents/passive.DOC</ref> therefore the balance of this radiation in the [[atmosphere]] is crucial to keep a stable [[temperature of the Earth|temperature]] and [[climate]]. [[Carbon dioxide]] in the atmosphere produces a [[greenhouse effect]], because CO<sub>2</sub> is able to absorb and re-emit infrared radiation as seen in Figure 2, unlike the gasses that make up most of the atmosphere ([[molecular oxygen]], O<sub>2</sub> about 21% and nitrogen, N<sub>2</sub>, about 78%).<ref name=UCAR>UCAR, ''Carbon Dioxide Absorbs and Re-emits Infrared Radiation'' [Online], Available: http://scied.ucar.edu/carbon-dioxide-absorbs-and-re-emits-infrared-radiation</ref> This greenhouse effect is [[temperature of the Earth without the greenhouse effect|necessary]] for the livable temperatures on Earth, however an increasing level of [[greenhouse gases]] is contributing to an unstable [[global warming|warming of the Earth]] which is a cause for great concern. Read more about this imbalance [[Earth's energy budget#Earth's Energy Imbalance|here]]. | ||
Since the infrared spectrum is of lower energy than visible light, this limits the amount of solar energy | Since the infrared spectrum is of lower energy than visible light, this limits the amount of solar energy that can be harnessed with standard [[photovoltaic cell]]s. | ||
==Use of IR== | ==Use of IR== | ||
[[File:Nightvision.jpg|200px|thumb|Figure 3. Night vision transforms infrared radiation emitted by objects into visible light that can be seen by the eye.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/5/52/Nightvision.jpg</ref>]] | [[File:Nightvision.jpg|200px|thumb|Figure 3. Night vision transforms infrared radiation emitted by objects into visible light that can be seen by the human eye.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/5/52/Nightvision.jpg</ref>]] | ||
Infrared radiation has many applications, some being: | Infrared radiation has many applications, some being: | ||
| Line 22: | Line 22: | ||
* Imaging (biological, mineral, defense, astronomy) | * Imaging (biological, mineral, defense, astronomy) | ||
* Climatology and meteorology | * Climatology and meteorology | ||
==For Further Reading== | |||
*[[Electromagnetic radiation]] | |||
*[[Heat]] | |||
*[[Radiant energy]] | |||
*[[Remote sensing]] | |||
*[[Light]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 16:10, 17 December 2018
Infrared radiation (IR) is a type of radiant energy, with longer wavelengths than the visible light humans can see, but shorter wavelengths than radio waves. Its range extends from fairly small wavelengths near the color red, 700x10-9 m, to nearly a millimeter, 3x10-4 m.[2]
Effect of IR
Even though infrared radiation cannot be seen by the human eye, it can definitely be felt. Infrared energy is felt as heat because it interacts with molecules by exciting them, causing them to move faster which increases the internal temperature of the object absorbing the infrared energy. Although all wavelengths of radiant energy will heat surfaces that absorb them, infrared radiation is most common in daily life because of the "ordinary" objects that emit it as radiant heat (see blackbody radiation and Wien's Law for more information on this).[3] For example, humans at a temperature of 37°C[4] emit most of their radiant heat in the infrared range, as can be seen in Figure 1.
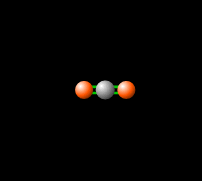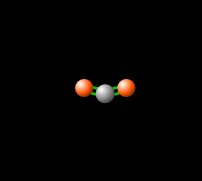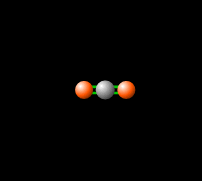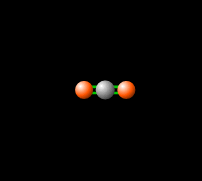
Around 50% of the Sun's energy to the Earth is in the form of infrared,[6] therefore the balance of this radiation in the atmosphere is crucial to keep a stable temperature and climate. Carbon dioxide in the atmosphere produces a greenhouse effect, because CO2 is able to absorb and re-emit infrared radiation as seen in Figure 2, unlike the gasses that make up most of the atmosphere (molecular oxygen, O2 about 21% and nitrogen, N2, about 78%).[7] This greenhouse effect is necessary for the livable temperatures on Earth, however an increasing level of greenhouse gases is contributing to an unstable warming of the Earth which is a cause for great concern. Read more about this imbalance here.
Since the infrared spectrum is of lower energy than visible light, this limits the amount of solar energy that can be harnessed with standard photovoltaic cells.
Use of IR

Infrared radiation has many applications, some being:
- Heating (cooking, saunas, industrial)
- Night vision (goggles, cameras)[9]
- Imaging (biological, mineral, defense, astronomy)
- Climatology and meteorology
For Further Reading
References
- ↑ Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/0/0c/Infrared_dog.jpg
- ↑ CRISP, Electromagnetic waves [Online], Available: http://www.crisp.nus.edu.sg/~research/tutorial/em.htm
- ↑ Hyperphysics, Heat Radiation [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/stefan.html#c2
- ↑ R. A. Hinrichs and M. Kleinbach, "Heat and Work," in Energy: Its Use and the Environment, 4th ed. Toronto, Ont. Canada: Thomson Brooks/Cole, 2006, ch.4, sec.E, pp.111-114
- ↑ PhET Simulations, Molecules and Light [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light
- ↑ Passive heating and cooling manual, Introduction to Solar Energy [Document], Available: http://www.azsolarcenter.com/design/documents/passive.DOC
- ↑ UCAR, Carbon Dioxide Absorbs and Re-emits Infrared Radiation [Online], Available: http://scied.ucar.edu/carbon-dioxide-absorbs-and-re-emits-infrared-radiation
- ↑ Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/5/52/Nightvision.jpg
- ↑ American Technologies Network Corporation, How Night Vision Works [Online], Available: http://www.atncorp.com/HowNightVisionWorks

