Methane: Difference between revisions
J.williams (talk | contribs) No edit summary |
m (1 revision imported) |
||
| (14 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-10-29]] [[Category:Translated to Spanish]] | ||
[[File:800px-Methane-3D-space-filling.svg.png|thumb| | [[Category: Translated to French]] | ||
[[fr:Méthane]] | |||
[[es:Metano]] | |||
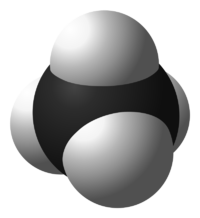
[[File:800px-Methane-3D-space-filling.svg.png|thumb|200px|Figure 1. Space filling model of methane; the white spheres represent [[hydrogen]] atoms and the black spheres represent [[carbon]] atoms.<ref> (2014, Dec. 12). ''Methane-3D-space-filling'' [Online]. Available: http://commons.wikimedia.org/wiki/File:Methane-3D-space-filling.svg#mediaviewer/File:Methane-3D-space-filling.svg</ref>]] | |||
<onlyinclude>'''Methane''' is a [[hydrocarbon]] | <onlyinclude>'''Methane''' is an [[alkane]] with the chemical formula CH<sub>4</sub>. As a [[hydrocarbon]], it can undergo [[hydrocarbon combustion]] which gives off [[heat]]. Methane is the main [[hydrocarbon]] component of [[natural gas]], which is a type of [[fossil fuel]].</onlyinclude><ref>“NATURAL GAS FAQs,” Pacific Northern Gas RSS. [Online]. Available: http://www.png.ca/natural-gas-faqs/. [Accessed: 24-May-2017]</ref> | ||
Methane [[combustion]] (see simulation at bottom of page) | At typical [[temperature]]s and [[pressure]]s it is a [[gas]] and makes up around 95% of the content of [[liquefied natural gas]], and around 80-90% of [[natural gas]].<ref>(2014, Jun. 10). ''Composition of Natural Gas and LNG'' [Online]. Available: http://www.beg.utexas.edu/energyecon/lng/LNG_introduction_07.php</ref> Methane is also a [[greenhouse gas]], like [[carbon dioxide]] (CO<sub>2</sub>). It has a shorter [[atmospheric lifetime]] than CO<sub>2</sub>, at 12 years,<ref name=book1>(2014, Jun. 10). ''Direct Global Warming Potentials'' [Online]. Available: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-10-2.html</ref> but this is "balanced" by the fact that it is more effective at trapping heat than CO<sub>2</sub>, as methane has a [[GWP]] ([[Global warming potential]]) of 21.<ref>''Direct Global Warming Potentials'' [Online]. Available: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-10-2.html [Accessed: 24-May-2017.</ref> | ||
Methane [[combustion]] (see simulation at bottom of page) provides a significant fraction of the world's [[primary energy]], and is used for [[home heating]], cooking food, heating [[water]], and [[electrical generation]]. It can even provide energy for [[transportation]]. However, this means that it is also a significant contributor to [[climate change]] since this methane produces a fair amount of the [[carbon dioxide]] that humans emit into the [[atmosphere]]. | |||
==Properties== | ==Properties== | ||
| Line 16: | Line 21: | ||
| Energy density<ref name=chemical>(2014, Dec. 12). ''Chemical Potential Energy'' [Online]. Available: http://physics.info/energy-chemical/</ref> || 55.5 MJ/kg | | Energy density<ref name=chemical>(2014, Dec. 12). ''Chemical Potential Energy'' [Online]. Available: http://physics.info/energy-chemical/</ref> || 55.5 MJ/kg | ||
|- | |- | ||
| Melting | | [[Melting point]] || -183<sup>o</sup>C<ref name=melting>(2015, Jan. 29). ''Boiling Points And Structures Of Hydrocarbons'' [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html</ref> | ||
|- | |- | ||
| Boiling | | [[Boiling point]] || -164<sup>o</sup>C<ref name=melting></ref> | ||
|- | |- | ||
| [[GWP]] || 21 | | [[GWP]] || 21 | ||
| Line 26: | Line 31: | ||
==Combustion Animation== | ==Combustion Animation== | ||
Methane | |||
<html><iframe src=' | Methane releases its [[chemical energy]] by undergoing [[hydrocarbon combustion]]. Below is a [[hydrocarbon combustion]] animation showing the net reaction that occurs when methane combines with [[oxygen]]. | ||
<center>CH<sub>4</sub> + 2O<sub>2</sub> → CO<sub>2</sub> + 2H<sub>2</sub>O Heat Energy ([[Enthalpy]]) </center> | |||
<html><iframe src='https://energyeducation.ca/simulations/combustion/combustion_methane.html' width='900px' height='330px' style='border:none;position:relative;left:-35px'></iframe></html> | |||
The [[hydrocarbon combustion]] reaction releases heat [[energy]] and is an example of an [[exothermic reaction]]. The reaction also has a negative [[enthalpy]] change (ΔH) value. | |||
==For Further Reading== | |||
*[[Chemical energy]] | |||
*[[Chemical bond]] | |||
*[[Combustion]] | |||
*[[Primary energy]] | |||
*[[Energy conversion technology]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 19:47, 20 December 2021
Methane is an alkane with the chemical formula CH4. As a hydrocarbon, it can undergo hydrocarbon combustion which gives off heat. Methane is the main hydrocarbon component of natural gas, which is a type of fossil fuel.[2]
At typical temperatures and pressures it is a gas and makes up around 95% of the content of liquefied natural gas, and around 80-90% of natural gas.[3] Methane is also a greenhouse gas, like carbon dioxide (CO2). It has a shorter atmospheric lifetime than CO2, at 12 years,[4] but this is "balanced" by the fact that it is more effective at trapping heat than CO2, as methane has a GWP (Global warming potential) of 21.[5]
Methane combustion (see simulation at bottom of page) provides a significant fraction of the world's primary energy, and is used for home heating, cooking food, heating water, and electrical generation. It can even provide energy for transportation. However, this means that it is also a significant contributor to climate change since this methane produces a fair amount of the carbon dioxide that humans emit into the atmosphere.
Properties
Below is a table of some of the basic properties of methane.
| Chemical formula | CH4 |
| Molar mass | 16.04 grams/mole |
| Energy density[6] | 55.5 MJ/kg |
| Melting point | -183oC[7] |
| Boiling point | -164oC[7] |
| GWP | 21 |
| Atmospheric lifetime | 12 years |
Combustion Animation
Methane releases its chemical energy by undergoing hydrocarbon combustion. Below is a hydrocarbon combustion animation showing the net reaction that occurs when methane combines with oxygen.
The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
For Further Reading
- Chemical energy
- Chemical bond
- Combustion
- Primary energy
- Energy conversion technology
- Or explore a random page
References
- ↑ (2014, Dec. 12). Methane-3D-space-filling [Online]. Available: http://commons.wikimedia.org/wiki/File:Methane-3D-space-filling.svg#mediaviewer/File:Methane-3D-space-filling.svg
- ↑ “NATURAL GAS FAQs,” Pacific Northern Gas RSS. [Online]. Available: http://www.png.ca/natural-gas-faqs/. [Accessed: 24-May-2017]
- ↑ (2014, Jun. 10). Composition of Natural Gas and LNG [Online]. Available: http://www.beg.utexas.edu/energyecon/lng/LNG_introduction_07.php
- ↑ (2014, Jun. 10). Direct Global Warming Potentials [Online]. Available: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-10-2.html
- ↑ Direct Global Warming Potentials [Online]. Available: http://www.ipcc.ch/publications_and_data/ar4/wg1/en/ch2s2-10-2.html [Accessed: 24-May-2017.
- ↑ (2014, Dec. 12). Chemical Potential Energy [Online]. Available: http://physics.info/energy-chemical/
- ↑ 7.0 7.1 (2015, Jan. 29). Boiling Points And Structures Of Hydrocarbons [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html