Methanol: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-06-15]] | ||
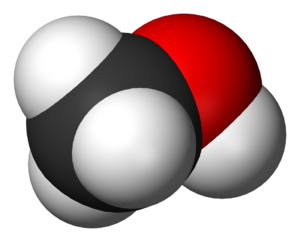
[[File:Methanol-3D-vdW.png|300px|thumbnail|right|Figure 1. Space filling model of methanol, the white is [[hydrogen]],black is [[carbon]], and the red is [[oxygen]].<ref>Ben Mills. (2015, Jan. 28). ''Methanol-3D-vdW'' [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanol-3D-vdW.png#mediaviewer/File:Methanol-3D-vdW.png</ref>]] | [[File:Methanol-3D-vdW.png|300px|thumbnail|right|Figure 1. Space filling model of methanol, the white is [[hydrogen]],black is [[carbon]], and the red is [[oxygen]].<ref>Ben Mills. (2015, Jan. 28). ''Methanol-3D-vdW'' [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanol-3D-vdW.png#mediaviewer/File:Methanol-3D-vdW.png</ref>]] | ||
| Line 24: | Line 24: | ||
==Combustion Animation== | ==Combustion Animation== | ||
Methanol is used as a combustible fuel. Below is an animation showing the net reaction that occurs during the [[hydrocarbon combustion]] of methanol. | Methanol is used as a combustible fuel. Below is an animation showing the net reaction that occurs during the [[hydrocarbon combustion]] of methanol. | ||
<html><iframe src=' | <html><iframe src='https://energyeducation.ca/simulations/combustion/combustion_methanol.html' width='900px' height='330px' style='border:none;position:relative;left:-35px'></iframe></html> | ||
==For Further Reading== | |||
*[[Chemical Energy]] | |||
*[[Chemical bond]] | |||
*[[Combustion]] | |||
*[[Primary energy]] | |||
*[[Energy conversion technology]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 21:09, 24 June 2018
Methanol is the simplest alcohol, and is also known as methyl alcohol. It is colourless, highly flammable, and at typical temperatures and pressures is a liquid. [2] Unlike ethanol, methanol cannot be ingested, as consumption may result in blindness, neurological damage, and damage to the nervous system.[3]
Methanol is used in the production of chemicals, as well as in the manufacturing of polyester fibers, acrylic plastics, and various pharmaceuticals. As well as being a major component in windshield washer fluid, methanol is used as an additive in gasoline.[4] When added to gasoline it serves as an antifreeze and octane booster to give the fuel a better, cleaner burn.
Like many hydrocarbon derivatives, methanol undergoes combustion (see simulation at bottom of page) when combined with heat and oxygen. This reaction releases energy, carbon dioxide, and water.
Properties
Below is a table of some of the basic properties of methanol.
| Chemical formula | CH3OH |
| Molar mass | 32.04 grams/mole [5] |
| Energy density[6] | 22.7 MJ/kg |
| Melting Point | -93.9oC[2] |
| Boiling Point | 64.96oC[2] |
Combustion Animation
Methanol is used as a combustible fuel. Below is an animation showing the net reaction that occurs during the hydrocarbon combustion of methanol.
For Further Reading
- Chemical Energy
- Chemical bond
- Combustion
- Primary energy
- Energy conversion technology
- Or explore a random page
References
- ↑ Ben Mills. (2015, Jan. 28). Methanol-3D-vdW [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanol-3D-vdW.png#mediaviewer/File:Methanol-3D-vdW.png
- ↑ 2.0 2.1 2.2 (2015, Jan. 28). Properties of Methanol [Online]. Available: http://www.methanol.org/technical-information/properties-of-methanol.aspx
- ↑ (2015, Jan. 28). Methanol Hazard Summary [Online]. Available: http://www.epa.gov/ttnatw01/hlthef/methanol.html
- ↑ (2015, Jan. 28). Methanol Principle Uses [Online]. Available: http://www.epa.gov/ttnatw01/hlthef/methanol.html
- ↑ (2015, Jan. 28). Physical Properties of Pure Methanol [Online]. Available: http://www.methanol.org/Technical-Information/Resources/Technical-Information/Physical-Properties-of-Pure-Methanol.aspx
- ↑ http://physics.info/energy-chemical/