Molecular oxygen
Molecular oxygen, or O2 is a diatomic molecule that is composed of two oxygen atoms held together by a covalent bond. Molecular oxygen is essential for life, since it's needed for respiration. It's also essential for fossil fuel combustion. Molecular oxygen is the chemically reactive part of the Earth's atmosphere, which means that oxygen is a chemical that wants to form chemical reactions with many different substances on Earth (see atmospheric oxygen for more details on this).
Molecular oxygen is used widely in the medical field for patients with breathing problems as well as in hyperbaric chambers. As well, it is used industrially to remove impurities such as sulfur from molten iron ore to produce steel. Molecular oxygen is also used widely in welding and metal cutting, and also acts as an oxidizer in rocket fuel.[2]
Properties
Below is a table of some of the basic properties of molecular oxygen.
| Chemical formula | O2 |
| Molar mass | 32.00 g/mol[3] |
| Boiling Point | -183oC[4] |
| Melting Point | −219°C[4] |
Combustion
Oxygen is a vital component of combustion, and is necessary for hydrocarbon combustion to occur. The combustion of hydrocarbons, especially in the context of burning fuels such as fossil fuels for energy, contributes greatly to climate change as a result of emissions such as carbon dioxide. Energy is obtained from fossil fuels through this combustion of the fuel, so having sufficient oxygen available is necessary for the production of energy.
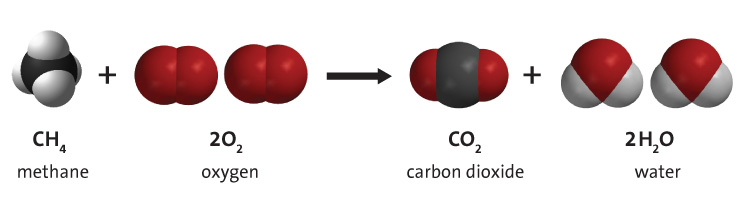
For combustion to occur, three components must exist. First, there must be some sort of fuel, generally a hydrocarbon, that actually burns. Second, there needs to be some amount of initial energy, known as activation energy, to get the reaction started. Finally, there needs to be a substance with an ability to accept the electrons that the fuel gives off when it is burned. This oxidizer is needed to ensure that charge is conserved. This substance is known as an oxidizer, and oxygen happens to be an extremely good oxidizer. Although fluorine is technically better at accepting these electrons, oxygen is more common and is easier to work with as fluorine violently reacts with many materials.[6] Thus oxygen is important to combustion as it fills this roll as oxidizer very well.
References
- ↑ Wikimedia Commons. (May 12, 2015). Dioxygen [Online]. Available: http://commons.wikimedia.org/wiki/File:Dioxygen-3D-vdW.png#/media/File:Dioxygen-3D-vdW.png
- ↑ WebElements. (May 12, 2015). Oxygen [Online]. Available: https://www.webelements.com/oxygen/uses.html
- ↑ Web QC. (May 13, 2015). Molar Mass of O2 [Online]. Available: http://www.webqc.org/molecular-weight-of-O2.html
- ↑ 4.0 4.1 Royal Society of Chemistry. (May 12, 2015). Molecular Oxygen [Online]. Available: http://www.chemspider.com/Chemical-Structure.952.html
- ↑ American Chemical Society. (June 8, 2015). "Methane and oxygen react" [Online]. Available: http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1
- ↑ Chemistry Stack Exchange. (June 8, 2015). Why is O2 the supporter of combustion? [Online]. Available: http://chemistry.stackexchange.com/questions/5708/why-is-o2-the-supporter-of-combustion

