Nucleus: Difference between revisions
No edit summary |
m (1 revision imported) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2021-01-31]] | ||
[[Category:Phets]] | [[Category:Phets]] | ||
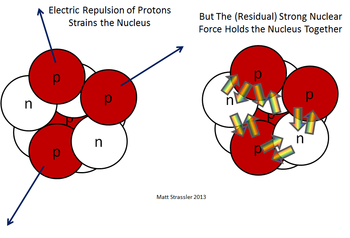
[[File:Nuclear force.png|350px|thumbnail|Figure 1. | [[File:Nuclear force.png|350px|thumbnail|Figure 1. On the left, the electrical force is pushing protons apart and, on the right, the strong force acting on both protons and neutrons inside a nucleus.<ref>Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014]. </ref>]] | ||
<onlyinclude>The '''nucleus''' is the central | <onlyinclude>The '''nucleus''' is the central and highly dense component of an [[atom]]. It is composed of [[proton]]s and [[neutron]]s, which are collectively called [[nucleon]]s.</onlyinclude> It accounts for the vast majority of an atom's [[mass]], however, due to the nature of [[electron]]s and the [[electromagnetic force]] that keeps the electrons orbiting the nucleus, only a small amount of an atoms size is attributed to the nucleus (the [[Atom#Composition|electron cloud]] accounts for most of the atom's size). | ||
==Holding the nucleus together== | ==Holding the nucleus together== | ||
There are two [[fundamental force]]s | There are two [[fundamental force]]s in the nucleus that aren't observed outside of nuclei. These forces are creatively named the [[strong force]] and the [[weak force]]. Tellingly, the [[strong force]] is extremely strong, in fact it's the strongest force known in the universe, but only acts over extremely small distances within the nucleus (about a [[meter|fm]] or 10<sup>-15</sup> m). It holds the nucleus together despite the intense [[electromagnetic force]] pushing apart the protons. | ||
The [[weak force]] is about 1,000,000 times weaker than the [[electromagnetic force]], but still quite a bit stronger than [[gravity]]. It balances the number of protons and neutrons within the nucleus by allowing [[beta decay]] | The [[weak force]] is about 1,000,000 times weaker than the [[electromagnetic force]], but still quite a bit stronger than [[gravity]]. It balances the number of protons and neutrons within the nucleus by allowing [[beta decay]], which converts protons into neutrons or neutrons into protons (depending on the type of beta decay). | ||
These two forces together allow the process of [[nuclear fusion]] to occur, which is essential to life | These two forces together allow the process of [[nuclear fusion]] to occur, which is essential to life since nuclear fusion powers the [[sun]]! They also allow the process of [[fission]] to occur, which is used to produce [[power]] for [[nuclear reactor]]s. Additionally, these forces govern what sorts of [[radioactive decay]] occur. These forces are both actively researched in physics. | ||
''To read more on how the nucleus is responsible for all the different [[element]]s please visit the page on [[atom]] | ''To read more on how the nucleus is responsible for all the different [[element]]s please visit the page on [[atom|atoms here]].'' | ||
== | ==Phet: Build a Nucleus== | ||
The [ | The [[who we are|Energy education team]] has adapted the following simulation from the [https://phet.colorado.edu/ University of Colorado]. This simulation shows how neutrons and protons sit in [[energy level]]s and [[Nuclear shell model|make up the nucleus]]. The number of neutrons and protons maintain particular ratios for the nucleus to be stable. | ||
<html><iframe src="https:// | <html><iframe src="https://energyeducation.ca/simulations/build-a-nucleus/build-a-nucleus_en.html" width="816" height="459"></iframe></html> | ||
==For Further Reading== | |||
*[[Atom]] | |||
*[[Electron]] | |||
*[[Neutron]] | |||
*[[Proton]] | |||
*[[Electromagnetic force]] | |||
*[[Alpha decay]] and [[Beta decay]] | |||
==References== | ==References== | ||
Latest revision as of 19:13, 15 October 2021
The nucleus is the central and highly dense component of an atom. It is composed of protons and neutrons, which are collectively called nucleons. It accounts for the vast majority of an atom's mass, however, due to the nature of electrons and the electromagnetic force that keeps the electrons orbiting the nucleus, only a small amount of an atoms size is attributed to the nucleus (the electron cloud accounts for most of the atom's size).
Holding the nucleus together
There are two fundamental forces in the nucleus that aren't observed outside of nuclei. These forces are creatively named the strong force and the weak force. Tellingly, the strong force is extremely strong, in fact it's the strongest force known in the universe, but only acts over extremely small distances within the nucleus (about a fm or 10-15 m). It holds the nucleus together despite the intense electromagnetic force pushing apart the protons.
The weak force is about 1,000,000 times weaker than the electromagnetic force, but still quite a bit stronger than gravity. It balances the number of protons and neutrons within the nucleus by allowing beta decay, which converts protons into neutrons or neutrons into protons (depending on the type of beta decay).
These two forces together allow the process of nuclear fusion to occur, which is essential to life since nuclear fusion powers the sun! They also allow the process of fission to occur, which is used to produce power for nuclear reactors. Additionally, these forces govern what sorts of radioactive decay occur. These forces are both actively researched in physics.
To read more on how the nucleus is responsible for all the different elements please visit the page on atoms here.
Phet: Build a Nucleus
The Energy education team has adapted the following simulation from the University of Colorado. This simulation shows how neutrons and protons sit in energy levels and make up the nucleus. The number of neutrons and protons maintain particular ratios for the nucleus to be stable.
For Further Reading
References
- ↑ Prof. Matt Strassler [Online]. Available: http://profmattstrassler.com/articles-and-posts/particle-physics-basics/the-structure-of-matter/the-nuclei-of-atoms-at-the-heart-of-matter/what-holds-nuclei-together/ [Accessed on 24 October 2014].

