Octane: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
m (1 revision imported) |
||
| (4 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2020-01-31]] | ||
[[File:N-octane-spaceFilling.png|thumb| | [[Category: Translated to French]] | ||
[[fr:Octane]] | |||
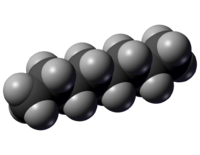
[[File:N-octane-spaceFilling.png|thumb|200px|Figure 1. Space filling model of octane, the grey spheres represent [[hydrogen]] atoms and the black spheres represents [[carbon]] atoms.<ref>"N-octane-spaceFilling" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:N-octane-spaceFilling.png#mediaviewer/File:N-octane-spaceFilling.png</ref>]] | |||
<onlyinclude>'''Octane''' is | <!--T:2--> | ||
<onlyinclude>'''Octane''' is an [[alkane]] with the chemical formula C<sub>8</sub>H<sub>18</sub>. As a [[hydrocarbon]], it can be burned as a [[fuel]]. | |||
This [[organic molecule]] is a colourless [[liquid]] at room [[temperature]] with a characteristic "gasoline" odour.</onlyinclude><ref>National Center for Biotechnology Information. (2015). ''Properties of Octane'' [Online]. Available: http://pubchem.ncbi.nlm.nih.gov/compound/octane#section=Top [February 16, 2015].</ref> | |||
Its principle use is as a component of [[gasoline]], and the performance of this gasoline depends on its [[octane rating]] which gets its name from this [[molecule]]. Note however, that the concept of octane rating is a little more complicated than just 'how much octane there is in the fuel'. The balanced chemical equation for the complete combustion of octane is: | |||
< | <chem>2C_8H_{18} + 25(O_2)\leftrightarrow 16(CO_2) + 18(H_2O)</chem> | ||
==Properties== <!--T:3--> | |||
<!--T:4--> | |||
Below is a table of some of the basic properties of octane. | Below is a table of some of the basic properties of octane. | ||
<!--T:5--> | |||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 26: | Line 30: | ||
|} | |} | ||
==Combustion Reaction== <!--T:6--> | |||
<!--T:7--> | |||
As is the case with other hydrocarbons, octane undergoes [[hydrocarbon combustion]], combining with [[oxygen]] to form [[carbon dioxide]]. The balanced chemical equation for the complete combustion of octane is: | |||
<!--T:8--> | |||
<center>2C<sub>8</sub>H<sub>18</sub> + 25O<sub>2</sub> → 16CO<sub>2</sub> + 18H<sub>2</sub>O + Heat Energy ([[Enthalpy]]) </center> | |||
<!--T:9--> | |||
The [[hydrocarbon combustion]] reaction releases [[heat]] [[energy]] and is an example of an [[exothermic reaction]]. The reaction also has a negative [[enthalpy]] change (ΔH) value. | |||
==For Further Reading== | |||
*[[Chemical energy]] | |||
*[[Chemical bond]] | |||
*[[Combustion]] | |||
*[[Primary energy]] | |||
*[[Energy conversion technology]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== <!--T:10--> | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 00:02, 27 September 2021
Octane is an alkane with the chemical formula C8H18. As a hydrocarbon, it can be burned as a fuel. This organic molecule is a colourless liquid at room temperature with a characteristic "gasoline" odour.[2] Its principle use is as a component of gasoline, and the performance of this gasoline depends on its octane rating which gets its name from this molecule. Note however, that the concept of octane rating is a little more complicated than just 'how much octane there is in the fuel'. The balanced chemical equation for the complete combustion of octane is:
Properties
Below is a table of some of the basic properties of octane.
| Formula | C8H18 |
| Molar mass | 114.23 grams/mole |
| Energy density | 47.9 MJ/kg [3] |
| Melting Point | -57oC[4] |
| Boiling Point | 125oC[4] |
Combustion Reaction
As is the case with other hydrocarbons, octane undergoes hydrocarbon combustion, combining with oxygen to form carbon dioxide. The balanced chemical equation for the complete combustion of octane is:
The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The reaction also has a negative enthalpy change (ΔH) value.
For Further Reading
- Chemical energy
- Chemical bond
- Combustion
- Primary energy
- Energy conversion technology
- Or explore a random page
References
- ↑ "N-octane-spaceFilling" Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:N-octane-spaceFilling.png#mediaviewer/File:N-octane-spaceFilling.png
- ↑ National Center for Biotechnology Information. (2015). Properties of Octane [Online]. Available: http://pubchem.ncbi.nlm.nih.gov/compound/octane#section=Top [February 16, 2015].
- ↑ Glenn Elert. (2015). The Physics Hypertextbook - Chemical Potential Energy [Online]. Available: http://physics.info/energy-chemical/ [February 16, 2015].
- ↑ 4.0 4.1 Charles E. Ophardt. (2003). Virtual Chembook - Hydrocarbon Boiling Points [Online]. Available: http://www.elmhurst.edu/~chm/vchembook/501hcboilingpts.html [February 16,2015].