Orbital: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
m (1 revision imported) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2020-02-29]] | ||
[[File:640px-F_orbital.png|400px|framed|right|Figure 1. Orbitals are | [[File:640px-F_orbital.png|400px|framed|right|Figure 1. Orbitals are complex shapes that are determined using quantum mechanics. Here are several orbital shapes that show where electrons are likely to be found. The numbers are one of the [[quantum number]]s used to describe these orbitals.<ref>Wikimedia Commons. (May 15, 2015). ''F Orbital'' [Online]. Available: http://commons.wikimedia.org/wiki/File:F_orbital.png#/media/File:F_orbital.png</ref>]] | ||
<onlyinclude>An '''orbital''' in an atom refers to the regions within the atom where electrons have the highest probability of being found, | <onlyinclude>[[Electron]]s are dispersed throughout an [[atom]] rather than being in one specific place. An '''orbital''' in an atom refers to the regions within the atom where electrons have the highest probability of being found. There are also nuclear orbitals, please see the [[nuclear shell model]] to learn more.</onlyinclude> In nuclear physics these orbitals are often referred to as the ''shell model'' or ''nuclear shells''. Determining ''why'' electrons prefer certain positions is a complex calculation in [[quantum mechanics]].<ref>J.Kotz, J.Townsend, P.Treichel. (May 15, 2015). Chemistry and Chemical Reactivity, 8th ed. Belmont, CA, U.S.A: Brooks/Cole, 2012.</ref> The [[energy]] required to remove an electron from an atom depends on which orbital the electron is in. | ||
A simplified [[model]] for orbitals describes spherical areas where electrons are more likely to be found — as "layers" in a cloud of electrons. A more complex quantum mechanical model describes these orbitals quite differently, including values known as '''[[quantum number]]s''', to describe the size and shape of these orbitals. An electron's orbital determines the [[energy]] that the electron has. Orbitals that result in two electrons having the same energy, while each electron is in a different orbital, are referred to as '''degenerate''' orbitals.<ref name="RE1"/> | |||
Although orbitals are complex, their structure | Although orbitals are complex, their structure explains many properties of [[matter]]. For example, the fullness of an orbital determines how that atom will [[chemical reaction|chemically react]], since the orbitals "want" to be full. For example, an [[element]] like [[helium]] is chemically unreactive, being a noble gas, its orbitals are full.<ref name="RE1">UC Davis Chemwiki. (May 15, 2015). ''Electronic Orbitals'' [Online]. Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals</ref> Many of the periodic properties of certain elements, and the properties that families of elements have, is caused by how these orbitals are filled with electrons.<ref>HyperPhysics. (May 15, 2015). ''Visualizing Electron Orbitals'' [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/eleorb.html</ref> | ||
To learn more about orbitals from a chemistry perspective, see [http://chemwiki.ucdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals UC Davis' Chemwiki]. | To learn more about orbitals from a chemistry perspective, see [http://chemwiki.ucdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals UC Davis' Chemwiki]. | ||
To learn more about orbitals from a physics perspective, see [http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/eleorb.html Hyperphysics]. | To learn more about orbitals from a physics perspective, see [http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/eleorb.html Hyperphysics]. The [[nuclear shell model]] describes how orbitals of [[proton]]s and [[neutron]]s inside of a [[nucleus]] determine nuclear properties in a similar way. | ||
==For Further Reading== | |||
*[[Electron]] | |||
*[[Valence and core electrons]] | |||
*[[Valence band]] | |||
*[[Nucleus]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 15:32, 28 April 2020
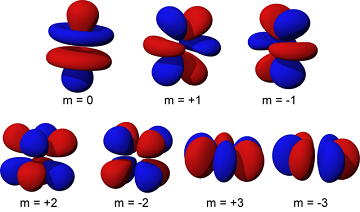
Electrons are dispersed throughout an atom rather than being in one specific place. An orbital in an atom refers to the regions within the atom where electrons have the highest probability of being found. There are also nuclear orbitals, please see the nuclear shell model to learn more. In nuclear physics these orbitals are often referred to as the shell model or nuclear shells. Determining why electrons prefer certain positions is a complex calculation in quantum mechanics.[2] The energy required to remove an electron from an atom depends on which orbital the electron is in.
A simplified model for orbitals describes spherical areas where electrons are more likely to be found — as "layers" in a cloud of electrons. A more complex quantum mechanical model describes these orbitals quite differently, including values known as quantum numbers, to describe the size and shape of these orbitals. An electron's orbital determines the energy that the electron has. Orbitals that result in two electrons having the same energy, while each electron is in a different orbital, are referred to as degenerate orbitals.[3]
Although orbitals are complex, their structure explains many properties of matter. For example, the fullness of an orbital determines how that atom will chemically react, since the orbitals "want" to be full. For example, an element like helium is chemically unreactive, being a noble gas, its orbitals are full.[3] Many of the periodic properties of certain elements, and the properties that families of elements have, is caused by how these orbitals are filled with electrons.[4]
To learn more about orbitals from a chemistry perspective, see UC Davis' Chemwiki.
To learn more about orbitals from a physics perspective, see Hyperphysics. The nuclear shell model describes how orbitals of protons and neutrons inside of a nucleus determine nuclear properties in a similar way.
For Further Reading
- Electron
- Valence and core electrons
- Valence band
- Nucleus
- Or explore a random page
References
- ↑ Wikimedia Commons. (May 15, 2015). F Orbital [Online]. Available: http://commons.wikimedia.org/wiki/File:F_orbital.png#/media/File:F_orbital.png
- ↑ J.Kotz, J.Townsend, P.Treichel. (May 15, 2015). Chemistry and Chemical Reactivity, 8th ed. Belmont, CA, U.S.A: Brooks/Cole, 2012.
- ↑ 3.0 3.1 UC Davis Chemwiki. (May 15, 2015). Electronic Orbitals [Online]. Available: http://chemwiki.ucdavis.edu/Physical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
- ↑ HyperPhysics. (May 15, 2015). Visualizing Electron Orbitals [Online]. Available: http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/eleorb.html

