Organic molecule: Difference between revisions
m (1 revision imported) |
m (1 revision imported) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category: Done 2021-01-31]] | ||
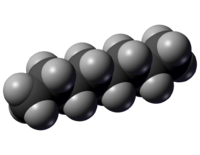
[[File:N-octane-spaceFilling.png|thumb|200px|Figure 1. Space-filling model of [[octane]]. The white spheres represent [[hydrogen]] atoms and the black spheres represent [[carbon]] atoms.<ref>"N-octane-spaceFilling" by Karlhahn - Own work. Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:N-octane-spaceFilling.png#mediaviewer/File:N-octane-spaceFilling.png</ref> Octane is an example of an '''organic molecule'''.]] | [[File:N-octane-spaceFilling.png|thumb|200px|Figure 1. Space-filling model of [[octane]]. The white spheres represent [[hydrogen]] atoms and the black spheres represent [[carbon]] atoms.<ref>"N-octane-spaceFilling" by Karlhahn - Own work. Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:N-octane-spaceFilling.png#mediaviewer/File:N-octane-spaceFilling.png</ref> Octane is an example of an '''organic molecule'''.]] | ||
<onlyinclude>'''Organic molecules''' are [[molecule]]s | <onlyinclude>'''Organic molecules''' are [[molecule]]s that are made of [[carbon]] ''and'' [[hydrogen]], and can include other elements.</onlyinclude> Organic molecules must contain carbon [[atom]]s covalently bonded to [[hydrogen]] atoms (C-H bonds). They usually involve [[oxygen]] and can also contain [[nitrogen]], [[sulfur]], [[phosphorous]], and others. [[Hydrocarbon]]s, like [[alkane]]s, [[alkene]]s and [[alkyne]]s are all organic molecules and so are [[alcohol]]s, carboxylic [[acid]]s and [[carbohydrate]]s. Many organic compounds are formed from chains of covalently-linked carbon atoms with hydrogen atoms attached to the chain (known as a hydrocarbon backbone).<ref>"Organic Compounds," LibreTexts, 2020. [Online]. Available: https://med.libretexts.org/@go/page/11106. [Accessed: 11_May-2021]</ref> | ||
Most (85% or so) of the world's [[primary energy]] comes from [[fossil fuel]]s, which are made up of mostly organic molecules. These organic molecules undergo [[combustion]] - they react with oxygen from the [[atmosphere]] and produce [[carbon dioxide]]. This process typically involves an [[exothermic reaction]] which releases heat energy that is converted into usable energy, usually with a [[heat engine]]. | Most (85% or so) of the world's [[primary energy]] comes from [[fossil fuel]]s, which are made up of mostly organic molecules. These organic molecules undergo [[combustion]] - they react with oxygen from the [[atmosphere]] and produce [[carbon dioxide]]. This process typically involves an [[exothermic reaction]] which releases heat energy that is converted into usable energy, usually with a [[heat engine]]. | ||
In addition to organic molecules found in [[fossil fuel]]s, organic molecules can be found in plant tissues, bacteria, and fungi. Other things like fruits and vegetables, wood, milk, paper and most plastics all contain organic compounds. In contrast, [[carbon dioxide]] (CO<sub>2</sub>) has carbon, but does not have hydrogen, thus, it is not an organic compound. Similarly, [[water]] (H<sub>2</sub>O) has hydrogen but no carbon, and is also not an organic compound. Generally, if a compound does not contain carbon '''and''' hydrogen atoms, it is considered '''inorganic'''.<ref>"Organic Compounds," LibreTexts, 2020. [Online]. Available: https://med.libretexts.org/@go/page/11106. [Accessed: 11_May-2021]</ref> | |||
Organic chemistry is a vast field that takes many years of study to fully understand; to get a start, please see [https://chem.libretexts.org/@go/page/225751 UC Davis's chem wiki]. | |||
==Further Reading== | |||
*[[Hydrocarbon]] | |||
*[[Fossil fuel]] | |||
*[[Chemical reaction]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
[[Category: Uploaded]] | |||
Latest revision as of 19:13, 15 October 2021
Organic molecules are molecules that are made of carbon and hydrogen, and can include other elements. Organic molecules must contain carbon atoms covalently bonded to hydrogen atoms (C-H bonds). They usually involve oxygen and can also contain nitrogen, sulfur, phosphorous, and others. Hydrocarbons, like alkanes, alkenes and alkynes are all organic molecules and so are alcohols, carboxylic acids and carbohydrates. Many organic compounds are formed from chains of covalently-linked carbon atoms with hydrogen atoms attached to the chain (known as a hydrocarbon backbone).[2]
Most (85% or so) of the world's primary energy comes from fossil fuels, which are made up of mostly organic molecules. These organic molecules undergo combustion - they react with oxygen from the atmosphere and produce carbon dioxide. This process typically involves an exothermic reaction which releases heat energy that is converted into usable energy, usually with a heat engine.
In addition to organic molecules found in fossil fuels, organic molecules can be found in plant tissues, bacteria, and fungi. Other things like fruits and vegetables, wood, milk, paper and most plastics all contain organic compounds. In contrast, carbon dioxide (CO2) has carbon, but does not have hydrogen, thus, it is not an organic compound. Similarly, water (H2O) has hydrogen but no carbon, and is also not an organic compound. Generally, if a compound does not contain carbon and hydrogen atoms, it is considered inorganic.[3]
Organic chemistry is a vast field that takes many years of study to fully understand; to get a start, please see UC Davis's chem wiki.
Further Reading
- Hydrocarbon
- Fossil fuel
- Chemical reaction
- Or explore a random page
References
- ↑ "N-octane-spaceFilling" by Karlhahn - Own work. Licensed under Public Domain via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:N-octane-spaceFilling.png#mediaviewer/File:N-octane-spaceFilling.png
- ↑ "Organic Compounds," LibreTexts, 2020. [Online]. Available: https://med.libretexts.org/@go/page/11106. [Accessed: 11_May-2021]
- ↑ "Organic Compounds," LibreTexts, 2020. [Online]. Available: https://med.libretexts.org/@go/page/11106. [Accessed: 11_May-2021]