Graphite

Graphite is a mineral composed of stacked sheets of carbon atoms with a hexagonal crystal structure. It is the most stable form of pure carbon under standard conditions. Graphite is very soft, has a low specific gravity, is relatively non-reactive, and has high electrical and thermal conductivity.[2]
Graphite occurs naturally in igneous and metamorphic rocks, where high temperatures and pressures compress carbon into graphite. Graphite can also be created synthetically by heating materials with high carbon content (e.g. petroleum coke or coal-tar pitch). The carbon-rich material is heated to 2500 to 3000 degrees Celsius, which is hot enough to "purify" the material of contaminants, allowing the carbon to form its hexagonal sheets.[3]
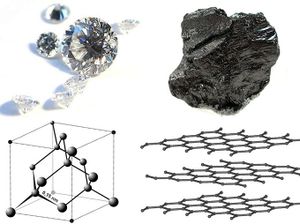
Graphite is extremely soft and breaks into thin flexible flakes that easily slide over one another, resulting in a greasy feel. Due to this, graphite is a good "dry" lubricant and can be used in applications where wet lubricants (like lubricating oil) cannot.[2]
Carbon has several other allotropes, or forms, that occur naturally, each with their own crystal structure(Figure 2). One form is graphene, which is a single layer of carbon atoms in a hexagonal pattern.[5] Another well-known allotrope of carbon, are diamonds. Although also composed of pure carbon, diamonds are almost entirely different in their physical properties.[6]
Uses
Graphite is used in a number of applications that require high temperatures and need a material that will not melt or disintegrate. Graphite is used to make the crucibles for the steel industry.[2] Graphite is also used as a neutron moderator in certain nuclear reactors, like the Soviet RBMK, due to its ability to slow down fast-moving neutrons.[7]
Other common uses of graphite include:[7]
- Pencil lead
- Lubricant
- Electrodes in batteries
- Brake linings for heavy vehicles
For Further Reading
- Mineral
- Nuclear reactor
- Carbon
- Atom
- Neutron
- Or explore a random page
References
- ↑ Wikimedia Commons.(Nov.28, 2018) " Grapite" [Online], Available: https://en.wikipedia.org/wiki/Graphite#/media/File:Graphite-233436.jpg
- ↑ 2.0 2.1 2.2 Minerals Education Coalition. (Accessed September 20, 2015). Graphite [Online], Available: https://www.mineralseducationcoalition.org/minerals/graphite
- ↑ King, H.M. (Nov. 28, 2018) "Graphite" [Online]. Available: https://geology.com/minerals/graphite.shtml
- ↑ WikiMedia Commons. (Accessed Nov. 28, 2018). File:Diamond and graphite2.jpg [Online], Available: https://en.wikipedia.org/wiki/Allotropes_of_carbon#/media/File:Diamond_and_graphite2.jpg
- ↑ Graphenea. (Accessed September 20, 2015). Graphene & Graphite [Online], Available: http://www.graphenea.com/pages/graphene-graphite#.Vf8hy_lViko
- ↑ Minerals.net. (Accessed September 20, 2015). The Mineral Graphite [Online], Available: http://www.minerals.net/mineral/graphite.aspx
- ↑ 7.0 7.1 P. Dutta. (Accessed September 20, 2015). What are the essential properties and uses of graphite ? [Online], Available: http://www.preservearticles.com/201012291918/properties-and-uses-of-graphite.html

