Chemical bond
Chemical bonds are the attractions between atoms that allow chemical compounds to form—molecules and crystals, like ionic solids and metals. Chemical bonds can also cause molecules to attract one another.
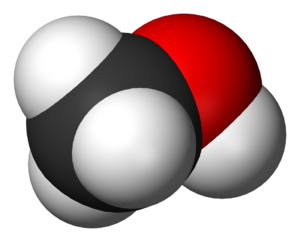
Figure 1 shows a molecule (methanol) that has chemical bonds holding the oxygen to the carbon and the hydrogen to the carbon and oxygen. If this molecule undergoes a chemical reaction, then these bonds will be broken and new bonds will be formed (see the simulation at the bottom of the page for an example). In order to break a chemical bond, energy must go into breaking the bond. Energy will also come out of forming new chemical bonds. The amount of energy needed to break a chemical bond is the same as the amount of energy that came from that chemical bond forming; this is an example of the conservation of energy.
Broadly speaking, chemical bonds occur when electrons are shared between two nuclei. The protons in each nucleus have a positive charge that attracts the electrons. Different bond types are determined by how the electrons are shared between and among different atoms:
- Covalent bond: electrons are shared in a molecule, as in H2 and O2.
- Ionic bond: an atom with a missing electron (such as Na+) bonds with an atom with an excess electron (such as Cl-). In this case, the NaCl, or table salt, is not a separate molecule, but part of a crystal.
- Metallic bonds: the valence electrons become shared in such a way that they are free to move.
To learn more please see UC Davis's chem wiki about covalent and ionic bonds, or metallic bonds.
The hydrogen bond is the most common example of an attraction between molecules. Many molecules with hydrogen, such as water, form an electric dipole, where the side of the molecule with the two hydrogen atoms is slightly positive, and the oxygen atom is slightly negative. When water molecules are near each other, such as in water, the oxygen in one molecule is attracted to the hydrogens in another. This is why water, a relatively light molecule, is a liquid at room temperature rather than a gas; this allows life as we know it to exist. To learn more about hydrogen bonds, please check out UC Davis's chemwiki.
The chemical energy that is released from chemical reactions comes from the energy in the chemical bonds. Forming strong bonds releases more energy, which means that it takes more energy to release the bonds. Weaker bonds have less energy associated with them. This is part of what makes carbon dioxide such a problem; carbon dioxide is in a very low energy state because of the strong bonds involved. This means that a fair amount of energy (from photosynthesis) is needed to break these bonds to make carbohydrates in plants. This is also why hydrocarbons (like biofuels or fossil fuels) are a good primary energy source.
Combustion Animation
Methanol is used as a combustible fuel. Below is an animation showing how the chemical bonds get broken for the oxygen molecule and the methanol. This leads to an overall release of chemical energy. This rearrangement of chemical bonds (chemical reaction) is called the hydrocarbon combustion of methanol.
References
- ↑ (2015, Feb. 6). Methanol-3D-vdW [Online]. Available: http://commons.wikimedia.org/wiki/File:Methanol-3D-vdW.png#mediaviewer/File:Methanol-3D-vdW.png