Ground level ozone: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
2dev>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2018-12-10]] | ||
[[File:Ozone-CRC-MW-3D-vdW.png|300px|thumb|Figure 1. Ozone (O<sub>3</sub>) consists of 3 [[oxygen]] atoms bonded together.<ref>"Ozone-CRC-MW-3D-vdW" by Ben Mills - Own work. Licensed under Public Domain via Wikimedia Commons - https://commons.wikimedia.org/wiki/File:Ozone-CRC-MW-3D-vdW.png#/media/File:Ozone-CRC-MW-3D-vdW.png</ref>]] | [[Category: Rudi grade Ashley edit]] | ||
[[File:Ozone-CRC-MW-3D-vdW.png|300px|thumb|Figure 1. Ozone (O<sub>3</sub>) consists of 3 [[oxygen]] atoms bonded together.<ref>"Ozone-CRC-MW-3D-vdW" by Ben Mills - Own work. Licensed under Public Domain via Wikimedia Commons - https://commons.wikimedia.org/wiki/File:Ozone-CRC-MW-3D-vdW.png#/media/File:Ozone-CRC-MW-3D-vdW.png</ref> While essential in the upper atmosphere, near the ground ozone causes breathing problems.]] | |||
<onlyinclude>'''Ground level ozone''' is a highly reactive [[secondary pollutant]] | <onlyinclude>'''Ground level ozone''' is a highly reactive [[secondary pollutant]]. This pollutant forms when [[primary pollutant]]s, like [[hydrocarbon]]s and [[NOx|nitrogen oxides]], react with [[sunlight]].</onlyinclude> Ozone irritates people's lungs and is a major component of [[photochemical smog]].<ref name=hackett>G. Tyler Miller, Jr. and D. Hackett, "Table 20-2 Major Outdoor Air Pollutants," in ''Living in the Environment'', 2nd ed. USA: Nelson , 2011, ch.20, sec.2, pp.464</ref> Ground level ozone gives photochemical smog its unpleasant odor and can cause lung function complications, especially in young children. | ||
==Formation== | ==Formation== | ||
Ground level ozone is created by chemical | Ground level ozone is created by a chemical reaction between [[volatile organic compound]]s and nitrogen oxides (Figure 1). The [[sun]] and high [[temperature]]s act as catalysts to this reaction.<ref name=hinrichs>R. A. Hinrichs and M. Kleinbach, "Nitrogen oxides, photochemical smog and ozone," in ''Energy: Its Use and the Environment'', 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2013, ch.8, sec.C, pp.250-252</ref> This makes its production within warm, densely populated, urban cities a common occurrence. However, since ozone takes some time to form, it will not necessarily stay within these cities. The pollutants may blow down-wind before ozone begins to form, therefore small towns and [[rural population]]s may also experience high levels of ozone (Figure 2).<Ref name=epa>US EPA. (Accessed July 27, 2015). ''Basic Information'' [Online], Available: http://www.epa.gov/ozonepollution/basic.html</ref> | ||
Although [[emissions]] of ozone-producing substances have decreased in recent years (see [[ground level ozone#Data Visualization|data visualization]] below) many urban areas do not yet meet air quality standards.<ref name=hinrichs/> | Although [[emissions]] of ozone-producing substances have decreased in recent years in Canada (see [[ground level ozone#Data Visualization|data visualization]] below) many urban areas do not yet meet air quality standards.<ref name=hinrichs/> | ||
The pollutants responsible for the formation of ozone are produced by [[hydrocarbon combustion]] in vehicles, factories and [[power plant]]s. Within cities | The pollutants responsible for the formation of ozone are produced by [[hydrocarbon combustion]] in vehicles, factories, and [[power plant]]s. Within cities, vehicles can make up nearly 90% of these pollutants.<Ref name=hinrichs/> Nitrogen oxides and VOCs are produced in the morning by cars traveling to work, and ozone is produced shortly after, typically in the afternoon. Since pollutants may flow down-wind from where they are produced, the ozone can find its way around the globe and be produced in safer quantities. However if there is an [[inversion layer]] present over the area of production, then the pollutants can be "trapped" over populated cities and form to unsafe levels in the form of smog.<ref name=hinrichs/> | ||
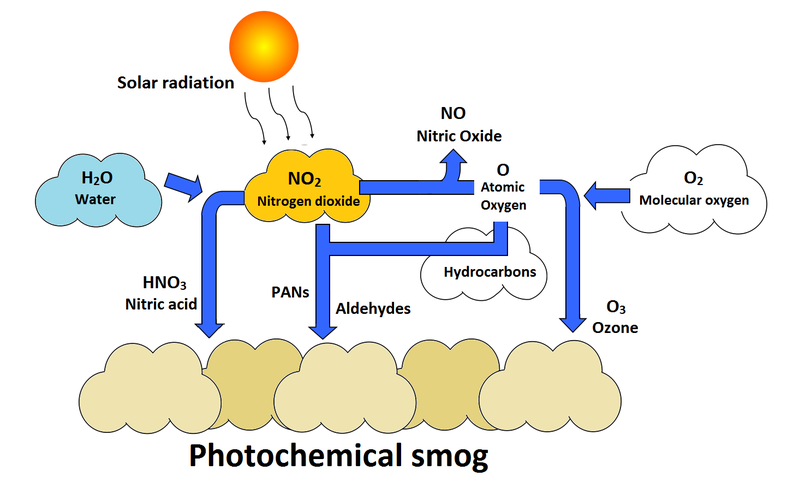
[[File:photochemsmog.png|thumb|800px|center|Figure 2. Ozone formation can be seen on the right, in which it is produced from photolyzed nitrogen dioxide.<ref>Adapted from ''Living In The Environment'': G. Tyler Miller, Jr. and D. Hackett, "Photochemical and Industrial Smog," in ''Living in the Environment'', 2nd ed. USA: Nelson , 2011, ch.20, sec.3, pp.465-471.</ref>]] | [[File:photochemsmog.png|thumb|800px|center|Figure 2. Ozone formation can be seen on the right, in which it is produced from photolyzed nitrogen dioxide.<ref>Adapted from ''Living In The Environment'': G. Tyler Miller, Jr. and D. Hackett, "Photochemical and Industrial Smog," in ''Living in the Environment'', 2nd ed. USA: Nelson , 2011, ch.20, sec.3, pp.465-471.</ref>]] | ||
| Line 15: | Line 16: | ||
==Health effects== | ==Health effects== | ||
Due to its reactivity and close proximity to | Due to its reactivity and close proximity to the Earth's surface, ground level ozone can have large effects on people. These effects include breathing problems, coughing, and irritation to the eyes, nose, and throat. People with asthma, bronchitis, emphysema, and heart disease may experience aggravated symptoms when exposed to ground level ozone. It can also reduce the body's immune system, increasing the potential for colds and flu. lung function, in particular, may be affected adversely.<Ref name=hackett/> | ||
Ground level ozone can affect the health of plants and animals. It can also cause damage to rubber, fabrics, and paints. <Ref name=hackett/> | |||
Visit the EPA to [http://www.epa.gov/ozonepollution/basic.html learn more] about ground level ozone. | Visit the EPA to [http://www.epa.gov/ozonepollution/basic.html learn more] about ground level ozone. | ||
==Data Visualization== | ==Data Visualization== | ||
The line graph below is used to show how levels of various emissions have been improving for Canada as a whole, particularly with efforts to scrub flue gases clean with various [[air pollution control devices]]. | The line graph below is used to show how levels of various emissions have been improving for Canada as a whole, particularly with efforts to scrub flue gases clean with various [[air pollution control devices]]. Ozone is a secondary pollutant, so its emissions cannot be tracked directly. Tracking the primary pollutants that create ozone gives some clue as to what is happening with ozone. NOx and VOCs have been decreasing, which are the two primary pollutants responsible for ozone formation. Please see the page on [[detailed pollution data]] for more extensive information on the Canadian release of these pollutants. | ||
<html> | <html> | ||
<iframe id='pollution-line' class='data-visualization pollutant-NOX'></iframe> | <iframe id='pollution-line' class='data-visualization pollutant-NOX'></iframe> | ||
</html> | </html> | ||
==For Further Reading== | |||
*[[Primary pollutant]] | |||
*[[Secondary pollutant]] | |||
*[[Air pollution control device]] | |||
*[[Emission]] | |||
*Or explore a [[Special:Random|random page]] | |||
==References== | ==References== | ||
{{Reflist}} | {{Reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Revision as of 21:22, 6 October 2018
Ground level ozone is a highly reactive secondary pollutant. This pollutant forms when primary pollutants, like hydrocarbons and nitrogen oxides, react with sunlight. Ozone irritates people's lungs and is a major component of photochemical smog.[2] Ground level ozone gives photochemical smog its unpleasant odor and can cause lung function complications, especially in young children.
Formation
Ground level ozone is created by a chemical reaction between volatile organic compounds and nitrogen oxides (Figure 1). The sun and high temperatures act as catalysts to this reaction.[3] This makes its production within warm, densely populated, urban cities a common occurrence. However, since ozone takes some time to form, it will not necessarily stay within these cities. The pollutants may blow down-wind before ozone begins to form, therefore small towns and rural populations may also experience high levels of ozone (Figure 2).[4]
Although emissions of ozone-producing substances have decreased in recent years in Canada (see data visualization below) many urban areas do not yet meet air quality standards.[3]
The pollutants responsible for the formation of ozone are produced by hydrocarbon combustion in vehicles, factories, and power plants. Within cities, vehicles can make up nearly 90% of these pollutants.[3] Nitrogen oxides and VOCs are produced in the morning by cars traveling to work, and ozone is produced shortly after, typically in the afternoon. Since pollutants may flow down-wind from where they are produced, the ozone can find its way around the globe and be produced in safer quantities. However if there is an inversion layer present over the area of production, then the pollutants can be "trapped" over populated cities and form to unsafe levels in the form of smog.[3]
Health effects
Due to its reactivity and close proximity to the Earth's surface, ground level ozone can have large effects on people. These effects include breathing problems, coughing, and irritation to the eyes, nose, and throat. People with asthma, bronchitis, emphysema, and heart disease may experience aggravated symptoms when exposed to ground level ozone. It can also reduce the body's immune system, increasing the potential for colds and flu. lung function, in particular, may be affected adversely.[2]
Ground level ozone can affect the health of plants and animals. It can also cause damage to rubber, fabrics, and paints. [2]
Visit the EPA to learn more about ground level ozone.
Data Visualization
The line graph below is used to show how levels of various emissions have been improving for Canada as a whole, particularly with efforts to scrub flue gases clean with various air pollution control devices. Ozone is a secondary pollutant, so its emissions cannot be tracked directly. Tracking the primary pollutants that create ozone gives some clue as to what is happening with ozone. NOx and VOCs have been decreasing, which are the two primary pollutants responsible for ozone formation. Please see the page on detailed pollution data for more extensive information on the Canadian release of these pollutants.
For Further Reading
- Primary pollutant
- Secondary pollutant
- Air pollution control device
- Emission
- Or explore a random page
References
- ↑ "Ozone-CRC-MW-3D-vdW" by Ben Mills - Own work. Licensed under Public Domain via Wikimedia Commons - https://commons.wikimedia.org/wiki/File:Ozone-CRC-MW-3D-vdW.png#/media/File:Ozone-CRC-MW-3D-vdW.png
- ↑ 2.0 2.1 2.2 G. Tyler Miller, Jr. and D. Hackett, "Table 20-2 Major Outdoor Air Pollutants," in Living in the Environment, 2nd ed. USA: Nelson , 2011, ch.20, sec.2, pp.464
- ↑ 3.0 3.1 3.2 3.3 R. A. Hinrichs and M. Kleinbach, "Nitrogen oxides, photochemical smog and ozone," in Energy: Its Use and the Environment, 5th ed. Toronto, Ont. Canada: Brooks/Cole, 2013, ch.8, sec.C, pp.250-252
- ↑ US EPA. (Accessed July 27, 2015). Basic Information [Online], Available: http://www.epa.gov/ozonepollution/basic.html
- ↑ Adapted from Living In The Environment: G. Tyler Miller, Jr. and D. Hackett, "Photochemical and Industrial Smog," in Living in the Environment, 2nd ed. USA: Nelson , 2011, ch.20, sec.3, pp.465-471.