Oil formation
Oil or petroleum is a readily combustable fossil fuel that is composed mainly of carbon and hydrogen, and is thus known as a hydrocarbon.[1] The formation of oil takes a significant amount of time with oil beginning to form millions of years ago. 70% of oil deposits existing today were formed in the Mesozoic age (252 to 66 million years ago), 20% were formed in the Cenozoic age (65 million years ago), and only 10% were formed in the Paleozoic age (541 to 252 million years ago). This is likely because the Mesozoic age was marked by a tropical climate, with large amounts of plankton in the ocean.[2]
The formation of oil begins in warm, shallow oceans that were present on the Earth millions of years ago. In these oceans, extremely small dead organic matter - classified as plankton - falls to the floor of the ocean. This plankton consists of animals, called zooplankton, or plants, called phytoplankton. This material then lands on the ocean floor and mixes with inorganic material that enters the ocean by rivers. It is this sediment on the ocean floor that then forms oil over many years. The energy in oil initially comes from the Sun, and is energy from sunlight that is trapped in chemical form by dead plankton.[3]
Formation Process
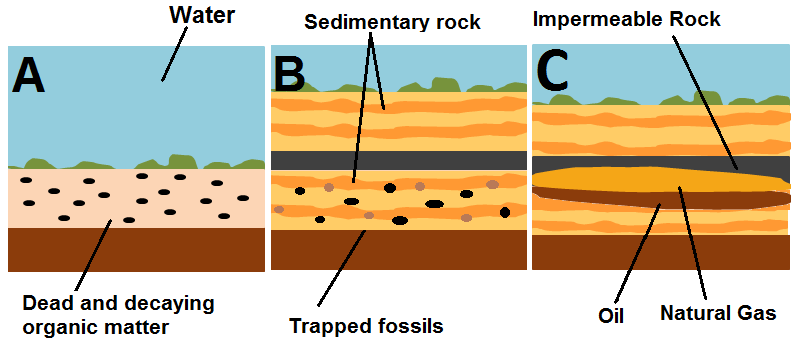
The process that creates oil is generally the same in most areas, although there may be different types of plant and animal debris that falls to the ocean floor and slightly different conditions. To form oil, the following steps have to happen:[3][5]
1. Dead plankton - both phytoplankton and zooplankton - as well as algae and bacteria sink to the bottom of an ancient ocean and mix with inorganic, clay-like materials that enter these oceans from streams and rivers. This creates an organic-rich mud. This mud can only form in still water environments. This step is shown in Figure 1, panel A.
2. This mud cannot be exposed to too much oxygen, or else the organic matter in the mud would be decomposed by bacteria and disappear quickly. Therefore environments where oil can form are known as anoxic environments. Before this organic matter is destroyed, it is buried by more sediment and lithifies (becomes sedimentary rock), creating organic shale. This step is shown in Figure 1, panel B. Burying material underwater is an easy way to create an anoxic environment because the atmosphere is not interacting with the decaying matter.
3. If this shale is buried between 2 and 4 kilometers, its temperature increases due to its location in the Earths interior. This increasing pressure and temperature of the shale transforms it into a waxy material known as kerogen. Shale that contains this material is known as oil shale.
4. If temperatures of the kerogen are greater than 90°C but lower than 160°C, the kerogen is transformed into oil and natural gas. At temperatures higher than this, only natural gas (literally a gas that's a hydrocarbon) or graphite is formed. This temperature range is known as the "oil window".
5. Oil is lighter than water, so as it escapes from the source oil shale it rises through pores in rocks, displacing water. Rock bodies that contain significant amounts of oil are known as reservoir rocks. For the oil to remain trapped in the reservoir, there must be some sort of thick, impermiable layer of rock to seal the reservoir. If this seal exists, then oil, gas, and water are trapped beneath and can be drilled into to obtain the oil.[6]
6. Geological changes in the Earth's crust bring these deposits up closer to the surface, making them somewhat easier to access.[7] This step is shown in Figure 1, panel C.
For Further Reading
- Oil
- Oil sands
- Shale
- Greenhouse effect
- Carbon capture and storage
- Or explore a random page
References
- ↑ Art Goldstein. (May 11, 2015). Formation of Oil [Online]. Available: http://f03.classes.colgate.edu/fsem037-oil/formation_of_oil.htm
- ↑ Colgate University. (January 7, 2016). Oil Formation [Online]. Available: http://f03.classes.colgate.edu/fsem037-oil/formation_of_oil.htm/
- ↑ 3.0 3.1 R. Wolfson. Energy, Environment and Climate, 2nd ed. New York, U.S.A.: Norton, 2012, pp. 96-97
- ↑ Created internally by a member of the Energy Education team
- ↑ Stephen Marshak. (May 11, 2015). Earth: Portrait of a Planet, 3rd ed. New York, NY, U.S.A:W.W. Norton & Company, 2008
- ↑ Canadian Federation of Earth Sciences. (May 11, 2015). Four Billion Years and Counting: Canada's Geological Heritage, 1st ed. Toronto, ON, Canada.: Nimbus Publishing, 2014
- ↑ J. Kraushaar, R. Ristinen. (May 11, 2015).Energy and the Environment, 2nd ed. Hoboken, NJ, U.S.A.: John Wiley & Sons, 2006, pp. 54

