Carbon dioxide: Difference between revisions
J.williams (talk | contribs) m (1 revision imported) |
No edit summary |
||
| Line 2: | Line 2: | ||
[[Category: Data Visualization]] | [[Category: Data Visualization]] | ||
[[category: Supporting page]] | [[category: Supporting page]] | ||
[[Category:Done | [[Category:Done 2016-01-15]] | ||
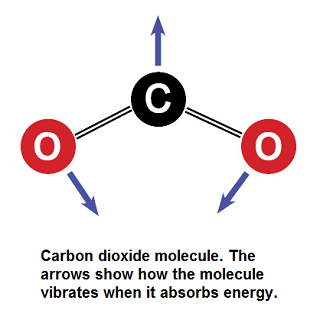
[[File:CO2_Drawing_Small.png|framed|right|Figure 1. Carbon dioxide with its vibration modes for absorbing infrared radiation<ref> Bredenberg, Al. (2015, June. 17). ''Carbon Dioxide -- How Can One Little Molecule Be Such a Big Troublemaker?''. [Online]. Available: http://news.thomasnet.com/IMT/2012/03/06/carbon-dioxide-how-can-one-little-molecule-be-such-a-big-troublemaker/</ref>]] | [[File:CO2_Drawing_Small.png|framed|right|Figure 1. Carbon dioxide with its vibration modes for absorbing infrared radiation<ref> Bredenberg, Al. (2015, June. 17). ''Carbon Dioxide -- How Can One Little Molecule Be Such a Big Troublemaker?''. [Online]. Available: http://news.thomasnet.com/IMT/2012/03/06/carbon-dioxide-how-can-one-little-molecule-be-such-a-big-troublemaker/</ref>]] | ||
[[File:co2gif.gif|318px|thumb|Figure 2. Carbon dioxide is able to interact with infrared radiation, leading to an imbalance of radiation entering and leaving the atmosphere.<ref>PhET Simulations, ''Molecules and Light'' [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light</ref>]] | [[File:co2gif.gif|318px|thumb|Figure 2. Carbon dioxide is able to interact with infrared radiation, leading to an imbalance of radiation entering and leaving the atmosphere.<ref>PhET Simulations, ''Molecules and Light'' [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light</ref>]] | ||
| Line 14: | Line 14: | ||
|} | |} | ||
Carbon dioxide (see Figure 1) is taken out of the [[atmosphere]] by [[photosynthesis]] | Carbon dioxide (see Figure 1) is taken out of the [[atmosphere]] by [[photosynthesis]] in plants to produce energy, and [[chemical]] uptake by the ocean (leading to [[ocean acidification]]). The total amount of carbon dioxide in the atmosphere has historically been in balance with the respiration of animals and plants. However, recently there has been a huge net increase in the levels of atmospheric and oceanic CO<sub>2</sub> because humans dig [[carbon]] (in the form of [[fossil fuel]]s) out of the ground and release it into the atmosphere. This major contribution of carbon dioxide into the atmosphere is as a byproduct of the [[hydrocarbon combustion|combustion]] of most [[fuel]]s. Chemically, carbon dioxide is one of the two main outputs in any form of combustion (the other being [[water]]). | ||
==Global Warming potential== | ==Global Warming potential== | ||
| Line 23: | Line 23: | ||
==Emissions== | ==Emissions== | ||
Carbon is the buzzword when talking about [[emissions]], and because of this it is important to know the difference between ''[[carbon]]'' and ''carbon dioxide''. Carbon is an element present in all organic material and is not, in itself, a bad thing. In fact, carbon is necessary to all life. Carbon dioxide, on the other hand, is what most people use when referring to "carbon emissions," it's a [[greenhouse gas]] and contributes to [[climate change]] because it helps to trap heat inside our atmosphere. Currently CO<sub>2</sub> levels in the oceans and atmosphere are on the rise, this increase can mostly be attributed to human activities<ref>IPCC. (2015, June. 17). ''Are the Increases in Atmospheric Carbon Dioxide and Other Greenhouse Gases During the Industrial Era Caused by Human Activities?'' [Online]. Available:http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-7-1.html</ref>, this is known as [[anthropogenic]] CO<sub>2</sub> emission. | Carbon is the buzzword when talking about [[emissions]], and because of this it is important to know the difference between ''[[carbon]]'' and ''carbon dioxide''. Carbon is an element present in all organic material and is not, in itself, a bad thing. In fact, carbon is necessary to all life. Carbon dioxide, on the other hand, is what most people use when referring to "carbon emissions," it's a [[greenhouse gas]] and contributes to [[climate change]] because it helps to trap heat inside our atmosphere. Currently CO<sub>2</sub> levels in the oceans and atmosphere are on the rise, this increase can mostly be attributed to human activities<ref>IPCC. (2015, June. 17). ''Are the Increases in Atmospheric Carbon Dioxide and Other Greenhouse Gases During the Industrial Era Caused by Human Activities?'' [Online]. Available:http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-7-1.html</ref>, this is known as [[anthropogenic emissions|anthropogenic]] CO<sub>2</sub> emission. | ||
Carbon dioxide emissions are often measured in [[Gigatonne]]s of carbon, or carbon dioxide. To convert from gigatons of carbon to gigatons of carbon dioxide, multiply by 44.01/12.01, or 3.664. The interactive graph below shows Megatonnes (1000 Megatonnes = 1 Gigatonne) of carbon dioxide emissions by country (or region depending on personal preference). | Carbon dioxide emissions are often measured in [[Gigatonne]]s of carbon, or carbon dioxide. To convert from gigatons of carbon to gigatons of carbon dioxide, multiply by 44.01/12.01, or 3.664. The interactive graph below shows Megatonnes (1000 Megatonnes = 1 Gigatonne) of carbon dioxide emissions by country (or region depending on personal preference). | ||
Revision as of 17:15, 9 February 2016
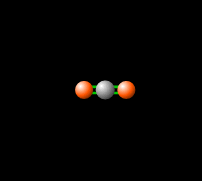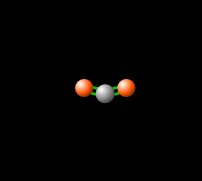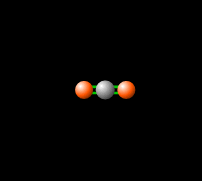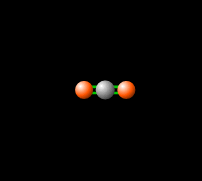
Carbon dioxide (CO2) is a naturally occurring gas, important to the carbon cycle for life and a byproduct of many forms of energy production. It is also a greenhouse gas.
| Chemical formula | CO2 |
| Molar mass | 44.01 grams/mole |
Carbon dioxide (see Figure 1) is taken out of the atmosphere by photosynthesis in plants to produce energy, and chemical uptake by the ocean (leading to ocean acidification). The total amount of carbon dioxide in the atmosphere has historically been in balance with the respiration of animals and plants. However, recently there has been a huge net increase in the levels of atmospheric and oceanic CO2 because humans dig carbon (in the form of fossil fuels) out of the ground and release it into the atmosphere. This major contribution of carbon dioxide into the atmosphere is as a byproduct of the combustion of most fuels. Chemically, carbon dioxide is one of the two main outputs in any form of combustion (the other being water).
Global Warming potential
The global warming potential (GWP) scale is a measurement of how much heat a greenhouse gas traps in the atmosphere. CO2 is used as a benchmark for this scale, hence carbon dioxide has a GWP value of 1. It is not easy to calculate how long carbon dioxide remains in the atmosphere after emission[3].
Carbon dioxide lets visible light through but its ability to vibrate on a molecular scale causes CO2 to absorb infrared radiation. This infrared radiation is what causes increased temperatures. Please see Figure 1 and 2 to see how the molecule vibrates to absorb, and re-emit infrared radiation.
Emissions
Carbon is the buzzword when talking about emissions, and because of this it is important to know the difference between carbon and carbon dioxide. Carbon is an element present in all organic material and is not, in itself, a bad thing. In fact, carbon is necessary to all life. Carbon dioxide, on the other hand, is what most people use when referring to "carbon emissions," it's a greenhouse gas and contributes to climate change because it helps to trap heat inside our atmosphere. Currently CO2 levels in the oceans and atmosphere are on the rise, this increase can mostly be attributed to human activities[4], this is known as anthropogenic CO2 emission.
Carbon dioxide emissions are often measured in Gigatonnes of carbon, or carbon dioxide. To convert from gigatons of carbon to gigatons of carbon dioxide, multiply by 44.01/12.01, or 3.664. The interactive graph below shows Megatonnes (1000 Megatonnes = 1 Gigatonne) of carbon dioxide emissions by country (or region depending on personal preference).
Scientists around the world are currently researching ways of capturing carbon and burying it underground in a process referred to as carbon capture and storage. If effective ways of doing this are developed, this would allow humanity to significantly reduce the atmospheric and oceanic CO2 levels and thus a prevent a dangerous rise in global temperature.
Interactive Graph
Select a graph type, and "compare by" group, and the chosen data will show in the box below.
References
- ↑ Bredenberg, Al. (2015, June. 17). Carbon Dioxide -- How Can One Little Molecule Be Such a Big Troublemaker?. [Online]. Available: http://news.thomasnet.com/IMT/2012/03/06/carbon-dioxide-how-can-one-little-molecule-be-such-a-big-troublemaker/
- ↑ PhET Simulations, Molecules and Light [Online], Available: https://phet.colorado.edu/en/simulation/molecules-and-light
- ↑ IPCC. (2015, June. 17) If Emissions of Greenhouse Gases are Reduced, How Quickly do Their Concentrations in the Atmosphere Decrease?. [Online]. Available:http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-10-3.html
- ↑ IPCC. (2015, June. 17). Are the Increases in Atmospheric Carbon Dioxide and Other Greenhouse Gases During the Industrial Era Caused by Human Activities? [Online]. Available:http://www.ipcc.ch/publications_and_data/ar4/wg1/en/faq-7-1.html

