Fuel cell: Difference between revisions
m (1 revision imported) |
energy>Jmdonev No edit summary |
||
| Line 1: | Line 1: | ||
[[category:371 topics]] | [[category:371 topics]] | ||
[[Category:Done | [[Category:Done 2020-02-29]] | ||
[[File:fuelcell.png|300px|thumb|Figure 1. A diagram of a hydrogen fuel cell. The hydrogen and oxygen combine to form water, while simultaneously producing electricity.<Ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Solid_oxide_fuel_cell.svg#/media/File:Solid_oxide_fuel_cell.svg</ref>]] | [[File:fuelcell.png|300px|thumb|Figure 1. A diagram of a hydrogen fuel cell. The hydrogen and oxygen combine to form water, while simultaneously producing electricity.<Ref>Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Solid_oxide_fuel_cell.svg#/media/File:Solid_oxide_fuel_cell.svg</ref>]] | ||
| Line 9: | Line 9: | ||
To produce electricity, a gaseous fuel is input and reacts with a catalyst made of [[platinum]] nanoparticles. When [[molecular hydrogen]] comes into contact with this, it splits into two H<sup>+</sup> ions and two [[electron]]s. The electrons are conducted through an [[electromotive force]] and electricity is produced. The hydrogen ions pass through a proton exchange membrane where it reaches the cathode and combines with [[oxygen]] to form water. This process can continue as long as there is hydrogen and oxygen supplied to the cell.<Ref name=hsw/> | To produce electricity, a gaseous fuel is input and reacts with a catalyst made of [[platinum]] nanoparticles. When [[molecular hydrogen]] comes into contact with this, it splits into two H<sup>+</sup> ions and two [[electron]]s. The electrons are conducted through an [[electromotive force]] and electricity is produced. The hydrogen ions pass through a proton exchange membrane where it reaches the cathode and combines with [[oxygen]] to form water. This process can continue as long as there is hydrogen and oxygen supplied to the cell.<Ref name=hsw/> | ||
The video below from NASA illustrates how oxygen and hydrogen come together in a fuel cell to release energy which can be snagged in a useful way. | |||
<html> | |||
<iframe width="560" height="315" src="https://www.youtube.com/embed/V3ChCroWttY" frameborder="0" allow="accelerometer; autoplay; encrypted-media; gyroscope; picture-in-picture" allowfullscreen></iframe> | |||
</html> | |||
===Hydrogen=== | ===Hydrogen=== | ||
| Line 21: | Line 24: | ||
[[File:Fuelcell.jpg|300px|thumb|Figure 2. A fuel cell vehicle configuration.<Ref>Wikimedia Commons. "Fuelcell" by Welleman, [Online], Available: https://commons.wikimedia.org/wiki/File:Fuelcell.jpg#/media/File:Fuelcell.jpg</ref>]] | [[File:Fuelcell.jpg|300px|thumb|Figure 2. A fuel cell vehicle configuration.<Ref>Wikimedia Commons. "Fuelcell" by Welleman, [Online], Available: https://commons.wikimedia.org/wiki/File:Fuelcell.jpg#/media/File:Fuelcell.jpg</ref>]] | ||
Many countries are spending enormous amounts of money into the development of hydrogen fuel cells | Many countries are spending enormous amounts of money into the development of hydrogen fuel cells. Burning [[oil product]]s (or any other [[fossil fuel]]s) produces [[pollution]]; most specifically, burning releases [[carbon dioxide]] which contributes to [[global warming]] and ultimately [[climate change|changing climate]].<Ref name=hsw/> | ||
Uses of fuel cells range from large electricity generating plants to small devices like [[cell phone]]s or laptops. | Uses of fuel cells range from large electricity generating plants to small devices like [[cell phone]]s or laptops. | ||
===In vehicles=== | ===In vehicles=== | ||
The most promising use for hydrogen as fuel (for the immediate future at least) are in vehicles—and there are already hydrogen based vehicles on the road today. The theoretical maximum efficiency of hydrogen fuel cells is 83%, | The most promising use for hydrogen as fuel (for the immediate future at least) are in vehicles—and there are already hydrogen based vehicles on the road today. The theoretical maximum efficiency of hydrogen fuel cells is 83%, with realistic efficiencies around 60%.<ref name=wolf>R. Wolfson, "Toward a Hydrogen Economy?" in ''Energy, Environment, and Climate'', 2nd ed., New York, NY: W.W. Norton & Company, 2012, ch. 11, sec. 2, pp. 307-315</ref> Combined with an [[electric motor]] of 90% efficiency, the vehicles can achieve an efficiency of 54% before factoring in other [[energy loss]]es. This is around 2 - 2.5 times higher than a typical [[gasoline]]-powered vehicle. | ||
There is plenty of criticism for these types of vehicles, as many claim the cost of these vehicles will never be practical. The | There is plenty of criticism for these types of vehicles, as many claim the cost of these vehicles will never be practical. The platinum in the fuel cells has made them expensive, but advances are bringing the price down.<ref name=hsw/> The durability and performance of such systems, along with the infrastructure necessary to accommodate them, is also under scrutiny. | ||
==For Further Reading== | ==For Further Reading== | ||
Revision as of 19:21, 10 April 2020
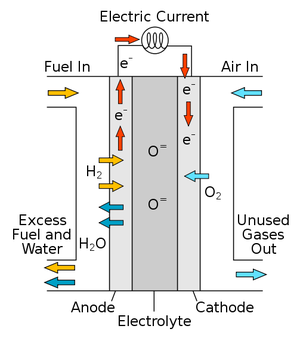
Fuel cells are a type of energy conversion technology which take the chemical energy contained within a fuel and transform it into electricity along with certain by-products (depending on the fuel used). [2] It's important to note that fuel cells are not heat engines, so they can have incredibly high efficiencies. However, when a heat engine is used to power a fuel cell, the heat engine still has a limiting thermal efficiency.
Fuel cells can be seen as an energy storage device, as energy can be input to create hydrogen and oxygen, which can remain in the cell until its use is needed at a later time. In this sense they work much like a battery.
To produce electricity, a gaseous fuel is input and reacts with a catalyst made of platinum nanoparticles. When molecular hydrogen comes into contact with this, it splits into two H+ ions and two electrons. The electrons are conducted through an electromotive force and electricity is produced. The hydrogen ions pass through a proton exchange membrane where it reaches the cathode and combines with oxygen to form water. This process can continue as long as there is hydrogen and oxygen supplied to the cell.[2]
The video below from NASA illustrates how oxygen and hydrogen come together in a fuel cell to release energy which can be snagged in a useful way.
Hydrogen
An attractive fuel for use in fuel cells is hydrogen, which is actively being researched and developed. The reasons for hydrogen use is not only because of its high energy density (121 MJ/kg),[3] but because the only by-product aside from electricity is water.
Although hydrogen is the most abundant element in the universe, it doesn't exist in its elemental form on Earth. Therefore hydrogen must be manufactured by inputting energy into hydrogen compounds like hydrocarbons or water. Depending on how this is done, the process may also release pollutants. Around 96% of hydrogen produced is done by using natural gas and other fossil fuels, resulting in considerable CO2 emissions. However, for the future, cleaner methods of attaining hydrogen (ie. water) are preferred.[2][4]
An alternative way to produce hydrogen comes from nuclear power. During off-peak periods (when the electrical grid does not require as much electricity) nuclear power plants may use their electricity for the production of hydrogen. The heat generated by the nuclear reactor could also be used for the production of hydrogen. Combined cycle plants producing both hydrogen and electricity may reach an efficiency of 60%.[5] Many Generation IV nuclear reactors are being designed with the purpose of hydrogen production.
Use of fuel cells
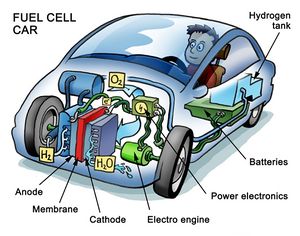
Many countries are spending enormous amounts of money into the development of hydrogen fuel cells. Burning oil products (or any other fossil fuels) produces pollution; most specifically, burning releases carbon dioxide which contributes to global warming and ultimately changing climate.[2]
Uses of fuel cells range from large electricity generating plants to small devices like cell phones or laptops.
In vehicles
The most promising use for hydrogen as fuel (for the immediate future at least) are in vehicles—and there are already hydrogen based vehicles on the road today. The theoretical maximum efficiency of hydrogen fuel cells is 83%, with realistic efficiencies around 60%.[7] Combined with an electric motor of 90% efficiency, the vehicles can achieve an efficiency of 54% before factoring in other energy losses. This is around 2 - 2.5 times higher than a typical gasoline-powered vehicle.
There is plenty of criticism for these types of vehicles, as many claim the cost of these vehicles will never be practical. The platinum in the fuel cells has made them expensive, but advances are bringing the price down.[2] The durability and performance of such systems, along with the infrastructure necessary to accommodate them, is also under scrutiny.
For Further Reading
- Thermal efficiency
- Hydrogen
- Alternative fuel vehicle
- Hydrogen as an energy currency
- Or explore a random page
References
- ↑ Wikimedia Commons [Online], Available: https://commons.wikimedia.org/wiki/File:Solid_oxide_fuel_cell.svg#/media/File:Solid_oxide_fuel_cell.svg
- ↑ 2.0 2.1 2.2 2.3 2.4 How Stuff Works. (August 10, 2015). How Fuel Cells Work [Online], Available: http://auto.howstuffworks.com/fuel-efficiency/alternative-fuels/fuel-cell.htm
- ↑ I. Hore-Lacy, "Future Energy Demand and Supply," in Nuclear Energy in the 21st Century, 2nd ed., London, UK: WNUP, 2011, ch.1, sec.6, pp.9
- ↑ G. Aubrecht. "Fuel Cells," in Energy: Physical, Environmental, and Social Impact, 3rd ed. San Francisco, CA, U.S.A.: Pearson, 2006, pp. 98-99
- ↑ I. Hore-Lacy, "Hydrogen Production and Use," in Nuclear Energy in the 21st Century, 2nd ed., London, UK: WNUP, 2011, ch.7, sec.2, pp.75-78
- ↑ Wikimedia Commons. "Fuelcell" by Welleman, [Online], Available: https://commons.wikimedia.org/wiki/File:Fuelcell.jpg#/media/File:Fuelcell.jpg
- ↑ R. Wolfson, "Toward a Hydrogen Economy?" in Energy, Environment, and Climate, 2nd ed., New York, NY: W.W. Norton & Company, 2012, ch. 11, sec. 2, pp. 307-315

