Oxygen: Difference between revisions
m (1 revision imported) |
m (1 revision imported) |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[Category:Done | [[Category:Done 2017-07-01]] | ||
[[Category:Translated to Spanish]] | |||
[[es:Oxígeno]] | |||
[[File:OXYGEN.png|200px|thumb|Figure 1. Oxygen, [[atomic number]] of 8 and [[atomic weight]] of 15.9994.<Ref>Made internally be a member of the Energy Education team, with information from periodictable.com, Available: http://periodictable.com/Elements/008/index.html</ref>]] | [[File:OXYGEN.png|200px|thumb|Figure 1. Oxygen, [[atomic number]] of 8 and [[atomic weight]] of 15.9994.<Ref>Made internally be a member of the Energy Education team, with information from periodictable.com, Available: http://periodictable.com/Elements/008/index.html</ref>]] | ||
<onlyinclude>'''Oxygen''' is the 8<sup>th</sup> [[element]] on the [[periodic table of elements|periodic table]], and is the 3<sup>rd</sup> most abundant element in the universe.<ref>Periodic Table, ''Abundance in the Universe'' [Online], Available: http://periodictable.com/Properties/A/UniverseAbundance.v.log.html</ref> Although the amount of oxygen in the universe is dwarfed by [[hydrogen]] and [[helium]], oxygen is the most common element on Earth, and it is a crucial component of life.</onlyinclude><ref>Periodic Table, ''Abundance in the Ocean'' [Online], Available: http://periodictable.com/Properties/A/OceanAbundance.v.log | <onlyinclude>'''Oxygen''' is the 8<sup>th</sup> [[element]] on the [[periodic table of elements|periodic table]], and is the 3<sup>rd</sup> most abundant element in the universe.<ref>Periodic Table, ''Abundance in the Universe'' [Online], Available: http://periodictable.com/Properties/A/UniverseAbundance.v.log.html</ref> Although the amount of oxygen in the universe is dwarfed by [[hydrogen]] and [[helium]], oxygen is the most common element on Earth, and it is a crucial component of life.</onlyinclude><ref>Periodic Table, ''Abundance in the Ocean'' [Online], Available: http://periodictable.com/Properties/A/OceanAbundance.v.log.html</ref> | ||
Oxygen exists in two elemental forms: [[molecular oxygen]] (O<sub>2</sub>) and [[ozone]] (O<sub>3</sub>). It also exists in countless compounds with other elements. Compounds of oxygen are called ''oxides''. Some examples of common oxides are minerals such as granite and quartz (oxides of silicon), rust (oxides of iron), and limestone (oxide of calcium and carbon). Many organic compounds also contain oxygen atoms in their structure. Read more about the chemistry of oxygen at the [https://chem.libretexts.org/Core/Inorganic_Chemistry/Descriptive_Chemistry/Elements_Organized_by_Block/2_p-Block_Elements/Group_16%3A_The_Oxygen_Family_(The_Chalcogens)/Z%3D008_Chemistry_of_Oxygen_(Z%3D8) Chemistry LibreText]. | |||
==Oxygen on Earth== | ==Oxygen on Earth== | ||
[[File:ocken.jpg|800px|center|thumb|Figure 2. Oxygen makes up a large part of the world we live in, both the ground and sky.<ref>Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/0/0d/Iss007e10807.jpg</ref>]] | |||
Oxygen makes up 46% of the [[cross section of the Earth|Earth's crust]], largely as ''silicates'', compounds of oxygen and [[silicon]]. <ref>Hyperphysics, ''Element Abundance in the Earth's Crust'' [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/tables/elabund.html</ref> Oxygen also comprises around 21% of the [[atmospheric oxygen|atmosphere]] as [[molecular oxygen]] and [[ozone]]. | |||
[[Ozone]] (O<sub>3</sub>) is a pollutant near the Earth's surface, but is a vital component of the [[layers of the atmosphere|stratosphere]], as it forms the [[ozone layer]] - a blanket of ozone that shields the planet's surface from [[ultraviolet radiation]]. Ozone forms naturally in the atmosphere, but can be destroyed by reaction with certain pollutants. In some areas, the depletion of ozone has created an "ozone hole" - a serious concern for the [[environment]].<ref>NASA, ''Ozone Hole Watch: Facts about ozone'' [Online], Available: http://ozonewatch.gsfc.nasa.gov/facts/SH.html</ref> | |||
Molecular oxygen (as O<sub>2</sub>) is essential for life. Many organisms require oxygen for metabolism, and [[photosynthesis|photosynthetic]] plants produce oxygen as they convert [[sunlight]] to stored [[energy]]. | |||
==Oxygen and Combustion== | |||
[[Molecular oxygen]] is important for [[combustion]] - especially in the combustion of [[fuel]]s for [[energy]]. Combustion is the reaction of a compound with an oxidant, which is usually molecular oxygen, to produce oxides. | |||
Combustion of [[fossil fuel]]s such as [[methane]], shown in Figure 3 below, produces [[carbon dioxide]], [[water vapour]], and energy. Fossil fuel combustion supplies around 95% of the world's [[primary energy]]. | |||
[[File:combustion_of_methane.jpg|800px|thumbnail|center|Figure | [[File:combustion_of_methane.jpg|800px|thumbnail|center|Figure 3. Methane reacting with oxygen to form carbon dioxide, water and heat.<ref name="image">American Chemical Society. "Methane and oxygen react". Internet: http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1, [October 25,2013]</ref>]] | ||
==Video== | ==Video== | ||
The video below is from the University of Nottingham's [http://www.periodicvideos.com/ | The video below is from the University of Nottingham's [http://www.periodicvideos.com/ Periodic Videos project].<ref>See more videos from the University of Nottingham on different elements here: http://www.periodicvideos.com/</ref> This video talks about oxygen, but also discusses [[ozone]]. | ||
<html> | <html> | ||
| Line 43: | Line 34: | ||
{{reflist}} | {{reflist}} | ||
[[Category:Uploaded]] | [[Category:Uploaded]] | ||
Latest revision as of 00:02, 27 September 2021
Oxygen is the 8th element on the periodic table, and is the 3rd most abundant element in the universe.[2] Although the amount of oxygen in the universe is dwarfed by hydrogen and helium, oxygen is the most common element on Earth, and it is a crucial component of life.[3]
Oxygen exists in two elemental forms: molecular oxygen (O2) and ozone (O3). It also exists in countless compounds with other elements. Compounds of oxygen are called oxides. Some examples of common oxides are minerals such as granite and quartz (oxides of silicon), rust (oxides of iron), and limestone (oxide of calcium and carbon). Many organic compounds also contain oxygen atoms in their structure. Read more about the chemistry of oxygen at the Chemistry LibreText.
Oxygen on Earth

Oxygen makes up 46% of the Earth's crust, largely as silicates, compounds of oxygen and silicon. [5] Oxygen also comprises around 21% of the atmosphere as molecular oxygen and ozone.
Ozone (O3) is a pollutant near the Earth's surface, but is a vital component of the stratosphere, as it forms the ozone layer - a blanket of ozone that shields the planet's surface from ultraviolet radiation. Ozone forms naturally in the atmosphere, but can be destroyed by reaction with certain pollutants. In some areas, the depletion of ozone has created an "ozone hole" - a serious concern for the environment.[6]
Molecular oxygen (as O2) is essential for life. Many organisms require oxygen for metabolism, and photosynthetic plants produce oxygen as they convert sunlight to stored energy.
Oxygen and Combustion
Molecular oxygen is important for combustion - especially in the combustion of fuels for energy. Combustion is the reaction of a compound with an oxidant, which is usually molecular oxygen, to produce oxides.
Combustion of fossil fuels such as methane, shown in Figure 3 below, produces carbon dioxide, water vapour, and energy. Fossil fuel combustion supplies around 95% of the world's primary energy.
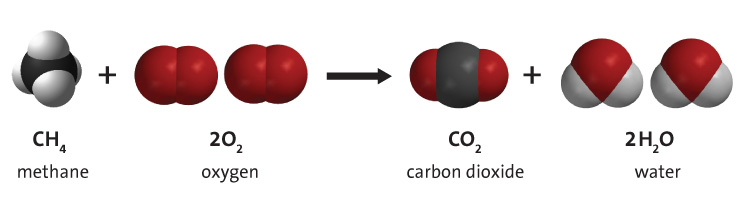
Video
The video below is from the University of Nottingham's Periodic Videos project.[8] This video talks about oxygen, but also discusses ozone.
References
- ↑ Made internally be a member of the Energy Education team, with information from periodictable.com, Available: http://periodictable.com/Elements/008/index.html
- ↑ Periodic Table, Abundance in the Universe [Online], Available: http://periodictable.com/Properties/A/UniverseAbundance.v.log.html
- ↑ Periodic Table, Abundance in the Ocean [Online], Available: http://periodictable.com/Properties/A/OceanAbundance.v.log.html
- ↑ Wikimedia Commons [Online], Available: http://upload.wikimedia.org/wikipedia/commons/0/0d/Iss007e10807.jpg
- ↑ Hyperphysics, Element Abundance in the Earth's Crust [Online], Available: http://hyperphysics.phy-astr.gsu.edu/hbase/tables/elabund.html
- ↑ NASA, Ozone Hole Watch: Facts about ozone [Online], Available: http://ozonewatch.gsfc.nasa.gov/facts/SH.html
- ↑ American Chemical Society. "Methane and oxygen react". Internet: http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1, [October 25,2013]
- ↑ See more videos from the University of Nottingham on different elements here: http://www.periodicvideos.com/


